

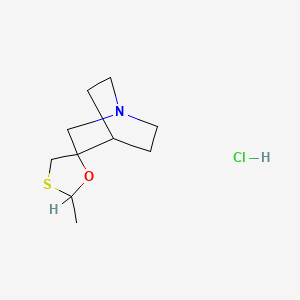

1. 2-methyspiro(1,3-oxathiolane-5,3)quinuclidine
2. Af 102b
3. Af 102b, (cis-(+))-isomer
4. Af 102b, (trans)-isomer
5. Af-102b
6. Af102b
7. Cevimeline
8. Cevimeline Hydrochloride
9. Evoxac
10. Fks 508
11. Fks-508
12. Sni 2011
13. Sni-2011
1. 173553-37-2
2. 107220-28-0
3. Cevimeline Hydrochloride
4. 2-methyl-1'-azaspiro[[1,3]oxathiolane-5,3'-bicyclo[2.2.2]octane] (hydrochloride)
5. Cevimeline Hcl
6. 2-methylspiro[1,3-oxathiolane-5,3'-1-azabicyclo[2.2.2]octane];hydrochloride
7. 2-methyl-1'-azaspiro[[1,3]oxathiolane-5,3'-bicyclo[2.2.2]octane] Hydrochloride
8. Spiro[1-azabicyclo[2.2.2]octane-3,5'-[1,3]oxathiolane], 2'-methyl-,hydrochloride
9. Af-102b
10. Schembl861315
11. Dtxsid701338114
12. Bcp12519
13. Cs-b1634
14. Yga55337
15. Akos037650732
16. Cs-15234
17. Ft-0664491
18. A925456
19. J-001739
20. (+/-)-cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine]
21. Cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine]hydrochloride
22. 2-methyl-1'-azaspiro[[1,3]oxathiolane-5,3'-bicyclo[2.2.2]octane] (hcl)
23. 5'-methyl-4-azaspiro[bicyclo[2.2.2]octane-2,2'-[1,4]oxathiolane] Hydrochloride
| Molecular Weight | 235.77 g/mol |
|---|---|
| Molecular Formula | C10H18ClNOS |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 235.0797631 g/mol |
| Monoisotopic Mass | 235.0797631 g/mol |
| Topological Polar Surface Area | 37.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 215 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Muscarinic Agonists
Drugs that bind to and activate muscarinic cholinergic receptors (RECEPTORS, MUSCARINIC). Muscarinic agonists are most commonly used when it is desirable to increase smooth muscle tone, especially in the GI tract, urinary bladder and the eye. They may also be used to reduce heart rate. (See all compounds classified as Muscarinic Agonists.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)