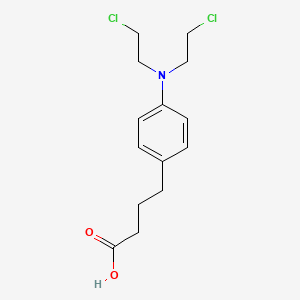

1. 4-(bis(2-chloroethyl)amino)benzenebutanoic Acid
2. Amboclorin
3. Cb 1348
4. Cb-1348
5. Cb1348
6. Chloraminophene
7. Chlorbutin
8. Leukeran
9. Lympholysin
10. N,n-di-(2-chloroethyl)-p-aminophenylbutyric Acid
11. Nsc 3088
12. Nsc-3088
13. Nsc3088
1. 305-03-3
2. Ambochlorin
3. Leukeran
4. Chloroambucil
5. Chloraminophen
6. Chlorbutin
7. Chloraminophene
8. Chlorobutine
9. Amboclorin
10. Ecloril
11. Chlorbutine
12. Chlorobutin
13. Lympholysin
14. Chlocambucil
15. Linfolizin
16. Linfolysin
17. Elcoril
18. Phenylbutyric Acid Nitrogen Mustard
19. Leukersan
20. Leukoran
21. 4-{4-[bis(2-chloroethyl)amino]phenyl}butanoic Acid
22. Chlorambucilum
23. Nsc-3088
24. Cb L348
25. Cb 1348
26. Phenylbuttersaeure-lost
27. Benzenebutanoic Acid, 4-[bis(2-chloroethyl)amino]-
28. Nsc 3088
29. Rcra Waste Number U035
30. Cb-1348
31. Nci-c03485
32. 4-[p-[bis(2-chloroethyl)amino]phenyl]butyric Acid
33. 4-(4-(bis(2-chloroethyl)amino)phenyl)butanoic Acid
34. 4-[4-[bis(2-chloroethyl)amino]phenyl]butanoic Acid
35. N,n-di-2-chloroethyl-gamma-p-aminophenylbutyric Acid
36. 4-(bis(2-chloroethyl)amino)benzenebutanoic Acid
37. P-(n,n-di-2-chloroethyl)aminophenyl Butyric Acid
38. 4-(p-bis(beta-chloroethyl)aminophenyl)butyric Acid
39. Benzenebutanoic Acid, 4-(bis(2-chloroethyl)amino)-
40. Chebi:28830
41. Nsc3088
42. 4-[bis(2-chloroethyl)amino]benzenebutyric Acid
43. 4-[bis(2-chloroethyl)amino]benzenebutanoic Acid
44. Chembl515
45. 4-(p-(bis(2-chloroethyl)amino)phenyl)butyric Acid
46. Gamma-[p-di(2-chloroethyl)aminophenyl]butyric Acid
47. Mls000028443
48. Chloorambucol
49. Chlorbutinum
50. Elcorin
51. 4-(bis(2-chloroethyl)amino)phenylbutyric Acid
52. Nci-3088
53. Gamma-(p-di(2-chloroethyl)aminophenyl)butyric Acid
54. Butyric Acid, 4-(p-bis(2-chloroethyl)aminophenyl)-
55. 18d0sl7309
56. Kyselina 4-(n,n-bis-(2-chlorethyl)-p-aminofenyl)maselna
57. Ncgc00015199-08
58. Clorambucile
59. Clorambucilo
60. Cas-305-03-3
61. Smr000058372
62. Clorambucile [dcit]
63. Dsstox_cid_263
64. Leukeran Tablets
65. 4-[bis(2-chloroethyl)amino]phenylbutyric Acid
66. Dsstox_rid_75472
67. Dsstox_gsid_20263
68. 4-(4-[bis(2-chloroethyl)amino]phenyl)butyric Acid
69. .gamma.-[p-di(2-chloroethyl)aminophenyl]butyric Acid
70. Butyric Acid, 4-[p-[bis(2-chloroethyl)amino]phenyl]-
71. Chlorambucilum [inn-latin]
72. Clorambucilo [inn-spanish]
73. P-n,n-di-(.beta.-chloroethyl)aminophenyl Butyric Acid
74. N,n-di-2-chloroethyl-.gamma.-p-aminophenylbutyric Acid
75. Ccris 126
76. Phenylbuttersaeure-lost [german]
77. Hsdb 3026
78. Sr-01000000062
79. Leukeran (tn)
80. 4-[4-[bis(2-chloroethyl)amino]phenyl]butyric Acid
81. Einecs 206-162-0
82. .gamma.-(p-bis(2-chloroethyl)aminophenyl)butyric Acid
83. .gamma.-[p-bis(2-chloroethyl)aminophenyl]butyric Acid
84. 4-(p-bis(.beta.-chloroethyl)aminophenyl)butyric Acid
85. 4-[p-bis(.beta.-chloroethyl)aminophenyl]butyric Acid
86. Rcra Waste No. U035
87. Butyric Acid, 4-(p-[bis(2-chloroethyl)amino]phenyl)-
88. Brn 0999011
89. Chlorambucilddv
90. Chlorambucil [usp:inn:ban]
91. Ai3-26083
92. Unii-18d0sl7309
93. P-(n,n-di-2-chlorethylaminophenyl)butyric Acid
94. Para-(di(2-chloroethyl)aminophenyl)butyric Acid
95. Chlorambucil,(s)
96. Gamma-(p-bis(2-chloroethyl)aminophenyl)butyricacid
97. Butanoic Acid, 4-(bis(2-chloroethyl)amino) Benzene
98. Gamma-(p-bis(2-chloroethyl)aminophenyl)butyric Acid
99. Phenyl)butanoic Acid
100. Mfcd00021783
101. P-n,n-di-(beta-chloroethyl)aminophenyl Butyric Acid
102. Butyric Acid, 4-(p-(bis(2-chloroethyl)amino)phenyl)
103. Para-n,n-di(beta-chloroethyl)aminophenyl Butyric Acid
104. N,n-di-2-chloroethyl-gamma-para-aminophenyl Butyric Acid
105. Opera_id_51
106. Spectrum_000118
107. Kyselina 4-(n,n-bis-(2-chlorethyl)-p-aminofenyl)maselna [czech]
108. Prestwick0_001079
109. Prestwick1_001079
110. Prestwick2_001079
111. Prestwick3_001079
112. Spectrum2_000065
113. Spectrum3_000336
114. Spectrum4_000273
115. Spectrum5_000677
116. Chlorambucil [mi]
117. Lopac-c-0253
118. Chlorambucil [inn]
119. Chlorambucil [jan]
120. Epitope Id:139977
121. Chlorambucil [hsdb]
122. Chlorambucil [iarc]
123. Schembl4308
124. Chlorambucil [vandf]
125. Chlorambucil With Impurity G
126. Lopac0_000227
127. Wln: Qv3r Dn2g2g
128. Bspbio_001098
129. Bspbio_001971
130. Chlorambucil [mart.]
131. Kbiogr_000766
132. Kbioss_000558
133. 4-14-00-01715 (beilstein Handbook Reference)
134. Mls001076130
135. Chlorambucil [usp-rs]
136. Chlorambucil [who-dd]
137. Chlorambucil [who-ip]
138. Divk1c_000688
139. Spectrum1500171
140. Chlorambucil (jan/usp/inn)
141. Spbio_000249
142. Spbio_002999
143. Bpbio1_001208
144. Gtpl7143
145. Zinc1115
146. Dtxsid7020263
147. Hms502c10
148. Kbio1_000688
149. Kbio2_000558
150. Kbio2_003126
151. Kbio2_005694
152. Kbio3_001191
153. Ninds_000688
154. Chlorambucil [orange Book]
155. Chlorambucil For System Suitability
156. Hms1571g20
157. Hms1920m15
158. Hms2090m19
159. Hms2091a22
160. Hms2098g20
161. Hms2235a04
162. Hms3259i10
163. Hms3372o04
164. Hms3652p08
165. Pharmakon1600-01500171
166. 4-(4-(bis(2-chloroethyl)amino)
167. Chlorambucil [ep Monograph]
168. Amy33445
169. Bcp28394
170. Chlorambucil [usp Monograph]
171. Tox21_110096
172. Tox21_201390
173. Tox21_302996
174. Bdbm50003677
175. Ccg-39872
176. Nsc756674
177. S4288
178. Chlorambucilum [who-ip Latin]
179. Akos024319346
180. Tox21_110096_1
181. Chlorambucil, Purum, >=98.0% (t)
182. Cs-3118
183. Db00291
184. Gs-6200
185. Lp00227
186. Nc00555
187. Nsc-756674
188. Sdccgsbi-0050215.p005
189. Idi1_000688
190. Ncgc00015199-01
191. Ncgc00015199-02
192. Ncgc00015199-03
193. Ncgc00015199-04
194. Ncgc00015199-05
195. Ncgc00015199-06
196. Ncgc00015199-07
197. Ncgc00015199-09
198. Ncgc00015199-10
199. Ncgc00015199-11
200. Ncgc00015199-12
201. Ncgc00015199-13
202. Ncgc00015199-14
203. Ncgc00015199-15
204. Ncgc00015199-16
205. Ncgc00015199-17
206. Ncgc00015199-19
207. Ncgc00015199-20
208. Ncgc00023250-00
209. Ncgc00023250-03
210. Ncgc00023250-04
211. Ncgc00023250-05
212. Ncgc00023250-06
213. Ncgc00023250-07
214. Ncgc00023250-08
215. Ncgc00023250-09
216. Ncgc00023250-10
217. Ncgc00256464-01
218. Ncgc00258941-01
219. Bp-24028
220. Hy-13593
221. Nci60_002639
222. Sbi-0050215.p004
223. Db-047794
224. Wr-139013
225. Ab00051938
226. Eu-0100227
227. Ft-0617365
228. Sw197258-4
229. A14252
230. A18607
231. C 0253
232. C06900
233. D00266
234. H10484
235. Ab00051938-14
236. Ab00051938-15
237. Ab00051938_16
238. 305c033
239. Q415939
240. 4-(4-(bis(2-chloroethyl)amino)phenyl)butanoicacid
241. 4[p-bis(.beta.-chloroethyl)aminophenyl]butyric Acid
242. Butanoic Acid, 4-(bis(2-chloroethyl)amino)benzene-
243. Sr-01000000062-2
244. Sr-01000000062-4
245. Sr-01000000062-7
246. W-106940
247. .gamma.-(p-bis(2-chloroethyl)aminophenyl)butyricacid
248. Brd-k29458283-001-04-2
249. Brd-k29458283-001-05-9
250. Brd-k29458283-001-17-4
251. 4-(4-[bis(2-chloroethyl)amino]phenyl)butanoic Acid #
252. Z1558572529
253. 4-[4-(n,n-bis(2-chloroethyl)-amino]phenyl)butanoic Acid
254. Chlorambucil, European Pharmacopoeia (ep) Reference Standard
255. Chlorambucil, United States Pharmacopeia (usp) Reference Standard
256. Chlorambucil For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 304.2 g/mol |
|---|---|
| Molecular Formula | C14H19Cl2NO2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 9 |
| Exact Mass | 303.0792842 g/mol |
| Monoisotopic Mass | 303.0792842 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 250 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Leukeran |
| PubMed Health | Chlorambucil (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | LEUKERAN (chlorambucil) was first synthesized by Everett et al. It is a bifunctional alkylating agent of the nitrogen mustard type that has been found active against selected human neoplastic diseases. Chlorambucil is known chemically as 4-[b... |
| Active Ingredient | Chlorambucil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Aspen Global |
| 2 of 2 | |
|---|---|
| Drug Name | Leukeran |
| PubMed Health | Chlorambucil (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | LEUKERAN (chlorambucil) was first synthesized by Everett et al. It is a bifunctional alkylating agent of the nitrogen mustard type that has been found active against selected human neoplastic diseases. Chlorambucil is known chemically as 4-[b... |
| Active Ingredient | Chlorambucil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Aspen Global |
Antineoplastic Agents, Alkylating; Carcinogens
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Chlorambucil is indicated in the treatment of chronic lymphatic (lymphocytic) leukemia, malignant lymphomas including lymphosarcoma, giant follicular lymphoma, and Hodgkin's disease. It is not curative in any of these disorders but may produce clinically useful palliation. /Include in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Chlorambucil is considered by many clinicians to be the drug of choice for the treatment of (Waldenstrom's) macroglobulinemia. /Not included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 981
Chlorambucil has also been used effectively with prednisone in the treatment of children with minimal-change nephrotic syndrome (lipoid nephrosis, idiopathic nephrotic syndrome of childhood) who have frequent relapses, require corticosteroid therapy to maintain remissions, or whose disease is resistant to steroid therapy. In most of these children, chlorambucil and prednisone therapy has induced long-term remissions and decreased the frequency of relapses. Although this type of nephrotic syndrome only occasionally occurs in adults, it is treated similarly. /Not included in US product label/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 981
For more Therapeutic Uses (Complete) data for CHLORAMBUCIL (9 total), please visit the HSDB record page.
/BOXED WARNING/ Chlorambucil can severely suppress bone marrow function. Chlorambucil is a carcinogen in humans. Chlorambucil is probably mutagenic and teratogenic in humans. Chlorambucil produces human infertility.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (December 2011). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Chlorambucil is contraindicated in patients with known hypersensitivity to the drug or in patients whose disease was resistant to prior therapy with the drug. The manufacturer states that there may be cross-sensitivity between chlorambucil and other alkylating agents manifested as rash. Chlorambucil should be discontinued promptly in patients who develop skin reactions.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 982
Adverse hematologic effects are the major and dose-limiting effects of chlorambucil. In usual doses, myelosuppression generally occurs gradually, is moderate in severity, and is usually reversible following discontinuance of the drug. Leukopenia, resulting from neutropenia and slowly progressive lymphopenia, occurs in many patients receiving chlorambucil. Thrombocytopenia and anemia may also occur.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 981
Chlorambucil appears to be relatively free of adverse GI effects unless single doses of 20 mg or more are administered. Adverse GI effects include nausea, vomiting, gastric discomfort or abdominal pain, anorexia, and diarrhea. Adverse GI effects are usually mild, last less than 24 hours, and disappear despite continued treatment; however, nausea and weakness have persisted up to 7 days in some patients following a single, high dose of the drug. If necessary, nausea and vomiting may usually be controlled with antiemetics. Oral ulceration has also been reported.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 981
For more Drug Warnings (Complete) data for CHLORAMBUCIL (25 total), please visit the HSDB record page.
For treatment of chronic lymphatic (lymphocytic) leukemia, childhood minimal-change nephrotic syndrome, and malignant lymphomas including lymphosarcoma, giant follicular lymphoma, Hodgkin's disease, non-Hodgkin's lymphomas, and Waldenstrms Macroglobulinemia.
Chlorambucil is an antineoplastic in the class of alkylating agents that is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L01AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AA - Nitrogen mustard analogues
L01AA02 - Chlorambucil
Route of Elimination
Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard. The pharmacokinetic data suggests that oral chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion.
Chlorambucil is rapidly and completely absorbed from the GI tract. Following single oral doses of 0.6-1.2 mg/kg, peak plasma concentrations of chlorambucil are reached within 1 hour.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 983
In a limited number of patients given a single oral dose of chlorambucil 0.2 mg/kg, an average peak plasma chlorambucil concentration of 492 ng/mL (adjusted to a dose of 12 mg) was reached at about 0.83 hours, and a mean peak plasma concentration of phenylacetic acid mustard (the major metabolite of chlorambucil) of 306 ng/mL (adjusted to a chlorambucil dose of 12 mg) occurred at approximately 1.9 hours. The area under the plasma concentration-time curve (AUC) of phenylacetic acid mustard was about 1.36 times greater than the AUC of chlorambucil.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 983
In a study of 12 patients given single oral doses of 0.2 mg/kg of chlorambucil, the mean dose (12 mg) adjusted (+/-SD) plasma chlorambucil Cmax was 492 +/- 160 ng/mL, the AUC was 883 +/- 329 ng.hr/mL, t1/2 was 1.3 +/- 0.5 hours, and the tmax was 0.83 +/- 0.53 hours. For the major metabolite, phenylacetic acid mustard, the mean dose (12 mg) adjusted (+/- SD) plasma Cmax was 306 +/- 73 ng/mL, the AUC was 1204 +/- 285 ng.h/mL, the t1/2 was 1.8 +/- 0.4 hours, and the tmax was 1.9 +/- 0.7 hours.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Chlorambucil and its metabolites are extensively bound to plasma and tissue proteins. In vitro, chlorambucil is 99% bound to plasma proteins, specifically albumin.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
For more Absorption, Distribution and Excretion (Complete) data for CHLORAMBUCIL (12 total), please visit the HSDB record page.
Chlorambucil undergoes rapid metabolism to phenylacetic acid mustard, the major metabolite, and the combined chlorambucil and phenylacetic acid mustard urinary excretion is extremely low - less than 1% in 24 hours.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Chlorambucil and its major metabolite spontaneously degrade in vivo forming monohydroxy and dihydroxy derivatives.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Chlorambucil is extensively metabolized in rodents by monochloroethylation and by beta oxidation, forming the phenylacetic acid derivative, which also has anticancer activity.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 123 (1981)
Ten metabolites of chlorambucil were isolated, most of which were phenylacetic acid & benzoic acid derivatives.
PMID:868079 MITOMA C ET AL; XENOBIOTICA 7 (4): 205-20 (1977)
1.5 hours
In a study of 12 patients given single oral doses of 0.2 mg/kg of chlorambucil, ... t1/2 was 1.3 +/- 0.5 hours, and the tmax was 0.83 +/- 0.53 hours. For the major metabolite, phenylacetic acid mustard, ... the t1/2 was 1.8 +/- 0.4 hours, and the tmax was 1.9 +/- 0.7 hours.
US Natl Inst Health; DailyMed. Current Medication Information for LEUKERAN (chlorambucil) tablet, film coated (November 2012). Available from, as of March 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6b2023e7-50dc-49c3-949d-1bc10ba256f0
Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases, preventing DNA synthesis and RNA transcription from the affected DNA, 2) DNA damage via the formation of cross-links (bonds between atoms in the DNA) which prevents DNA from being separated for synthesis or transcription, and 3) the induction of mispairing of the nucleotides leading to mutations.
As an alkylating agent, chlorambucil interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function. In vitro studies have shown that the major metabolite of chlorambucil (phenylacetic acid mustard), which is also a bifunctional alkylating compound, has antineoplastic activity against some neoplastic human cell lines that is approximately equal to that of chlorambucil. Therefore, the major metabolite of chlorambucil may contribute to the in vivo antitumor activity of the drug. Chlorambucil also possesses some immunosuppressive activity, principally due to its suppression of lymphocytes. The drug is the slowest acting and generally least toxic of the presently available nitrogen mustard derivatives.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 983
A marked transient increase was observed in ribonucleotide reductase activity within 2 hr of exposing BALB/c 3T3 mouse cells to DNA damaging concentrations of chlorambucil. Elevations in activity were accompanied by transient increases in the mRNA levels of both genes (R1 and R2) that code for ribonucleotide reductase. Only the protein for the limiting component for enzyme activity R2 was significantly elevated in chlorambucil treated cultures. The chlorambucil effects upon activity and regulation of ribonucleotide reductase occurred without any detectable changes in the rate of DNA synthesis, as would be expected if the elevation in enzyme activity is required for DNA repair. The chlorambucil-induced elevations in R1 and R2 message levels were blocked by treatment of cells with actinomycin D or the tumor promoter 12-O-tetradecanoylphorbol-13-acetate indicating the importance of the reductase transcriptional process in responding to the action of chlorambucil and providing evidence for the involvement of a protein kinase C pathway in the regulation of mammalian ribonucleotide reductase. In addition to the chlorambucil-induced elevations in enzyme activity, message, and protein levels, the drug was also shown to be an inhibitor of ribonucleotide reductase activity in cell-free preparations. Both R1 and R2 proteins were targets for chlorambucil, in keeping with the known alkylating abilities of the drug.
PMID:1551913 Hurta RA, Wright JA; J Biol Chem 267 (10): 7066-71 (1992)
/ALTERNATIVE and IN VITRO TESTS/ Reaction of one of the two chloroethyl groups of chlorambucil with the N7 position of guanine or adenine of double-stranded DNA leads to the formation of mono-adducts. These are repaired rapidly in an error-free fashion by methylguanine methyltransferase (sometimes called alkylguanine alkyltransferase). However, some cells lack this repair activity, usually because of silencing of the corresponding gene, and the unrepaired DNA mono-adduct then forms a complex with mismatch-repair enzymes. The subsequent inhibition of DNA replication can eventually induce DNA breakage. The second chloroethyl group of the DNA mono-adduct with chlorambucil can interact with proteins but more importantly, because of its juxtaposition to other bases in the major groove of DNA, it can react with a DNA base to form an interstrand DNA cross-link. This DNA crosslink complex is quite stable, and its repair requires nucleotide excision repair factors (such as xeroderma pigmentosum complementation group F-excison repair cross-complementing rodent repair deficiency, complementation group, 1-XPF-ERCC1) that act slowly by homologous recombination. The DNA cross-link attracts several binding proteins, probably the BRCA1 and BRCA2 proteins, Fanconi anemia gene product, and Nijmegen breakage syndrome gene product to form a complex. As shown in cultured HeLa cells, addition of chlorambucil prolongs S-phase and induces a corresponding mitotic delay. The magnitude of these effects correlates with the level of DNA cross-links. Treatment of cells in the G2-phase of the cell cycle does not induce mitotic delay but does inhibit DNA synthesis in the subsequent cell cycle, and causes a delay in the next mitosis, suggesting that at least some lesions induced by chlorambucil are long-lasting.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V100A 52 (2012)