

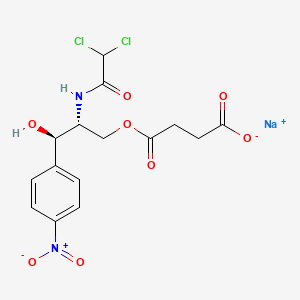

1. Chloramphenicol Hemisuccinate
2. Chloramphenicol Monosuccinate
3. Chloramphenicol Succinate Sodium
4. Levomycetin Succinate
1. 982-57-0
2. Chloramphenicol Succinate Sodium
3. Chloramphenicol Monosuccinate Sodium Salt
4. Chloramphenicol Succinate Sodium
5. 872109hx6b
6. Protophenicol
7. Dsstox_cid_4747
8. Dsstox_rid_77520
9. Dsstox_gsid_24747
10. Butanedioic Acid, Mono(2-((2,2-dichloroacetyl)amino)-3-hydroxy-3-(4-nitrophenyl)propyl) Ester, Monosodium Salt, (r-(r*,r*))-
11. Sodium Chloramphenicol Succinate
12. Chloramphenicol Sodium Monosuccinate
13. Cas-982-57-0
14. Sodium 4-((2r,3r)-2-(2,2-dichloroacetamido)-3-hydroxy-3-(4-nitrophenyl)propoxy)-4-oxobutanoate
15. Ccris 6204
16. Chloramphenicol-sukzinat-natrium
17. Ncgc00094619-04
18. Chloramphenicol Sodium Succinate, D-
19. Einecs 213-568-1
20. Chloramphenicol-sukzinat-natrium [german]
21. Unii-872109hx6b
22. Sodium;4-[(2r,3r)-2-[(2,2-dichloroacetyl)amino]-3-hydroxy-3-(4-nitrophenyl)propoxy]-4-oxobutanoate
23. Chloramphenicol Sodium Succinate [usp:ban:jan]
24. Chloromycetin Succinate (tn)
25. D-threo-(-)-2,2-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide Alpha-(sodium Succinate)
26. Schembl193134
27. Chloramphenicoli Natrii Succinas
28. Chembl1200729
29. Dtxsid2024747
30. Tox21_113484
31. Akos016340429
32. Tox21_113484_1
33. Ccg-212521
34. Ks-5158
35. Ncgc00094619-06
36. Acetamide, 2,2-dichloro-n-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)-, Alpha-ester With Sodium Succinate
37. Butanedioic Acid, Mono(2-((2,2-dichloroacetyl)amino)-3-hydroxy-3-(4-nitrophenyl)propyl) Ester, Monosodium Salt, (r-(r(sup *),r(sup *)))(-)
38. Chloramphenicol Sodium Succinate (jp17/usp)
39. Chloramphenicol Sodium Succinate [jan]
40. Chloramphenicol Sodium Succinate [vandf]
41. D02185
42. Chloramphenicol Sodium Succinate [mart.]
43. Chloramphenicol Sodium Succinate [who-dd]
44. Chloramphenicol Sodium Succinate [who-ip]
45. W-100087
46. Chloramphenicol Monosuccinate Sodium Salt [mi]
47. Chloramphenicol Sodium Succinate [ep Monograph]
48. Chloramphenicol Sodium Succinate [orange Book]
49. Chloramphenicoli Natrii Succinas [who-ip Latin]
50. Q27269783
51. Chloramphenicol Sodium Succinate [usp Monograph]
52. Butanedioic Acid, Mono((2r,3r)-2-((dichloroacetyl)amino)-3-hydroxy-3-(4-nitrophenyl)propyl) Ester, Monosodium Salt
53. D-threo-(-)-2,2-dichloro-n-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl)acetamide .alpha.-(sodium Succinate)
54. Sodium4-((2r,3r)-2-(2,2-dichloroacetamido)-3-hydroxy-3-(4-nitrophenyl)propoxy)-4-oxobutanoate
| Molecular Weight | 445.2 g/mol |
|---|---|
| Molecular Formula | C15H15Cl2N2NaO8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 444.0103151 g/mol |
| Monoisotopic Mass | 444.0103151 g/mol |
| Topological Polar Surface Area | 162 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 541 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Chloramphenicol sodium succinate |
| Drug Label | IMPORTANT CONSIDERATIONS IN PRESCRIBING INJECTABLE CHLORAMPHENICOL SODIUM SUCCINATE.CHLORAMPHENICOL SODIUM SUCCINATE IS INTENDED FOR INTRAVENOUS USE ONLY. IT HAS BEENDEMONSTRATED TO BE INEFFECTIVE WHEN GIVEN INTRAMUSCULARLY.1. Chloramphenicol sodium... |
| Active Ingredient | Chloramphenicol sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1gm base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Chloramphenicol sodium succinate |
| Drug Label | IMPORTANT CONSIDERATIONS IN PRESCRIBING INJECTABLE CHLORAMPHENICOL SODIUM SUCCINATE.CHLORAMPHENICOL SODIUM SUCCINATE IS INTENDED FOR INTRAVENOUS USE ONLY. IT HAS BEENDEMONSTRATED TO BE INEFFECTIVE WHEN GIVEN INTRAMUSCULARLY.1. Chloramphenicol sodium... |
| Active Ingredient | Chloramphenicol sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1gm base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)