

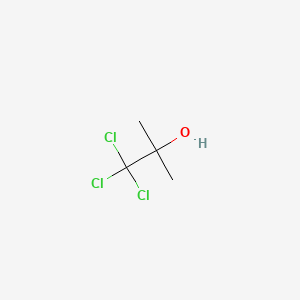

1. Acetonchloroform
2. Anhydrous Chlorobutanol
3. Chlorbutol
4. Chloretone
5. Chlorobutanol, Anhydrous
6. Trichlorbutanol
1. 57-15-8
2. Chlorbutol
3. Chloretone
4. 1,1,1-trichloro-2-methyl-2-propanol
5. 1,1,1-trichloro-2-methylpropan-2-ol
6. Chloreton
7. Chlorbutanol
8. Acetonchloroform
9. Acetone Chloroform
10. Acetochlorone
11. Chlortran
12. Anhydrous Chlorobutanol
13. Coliquifilm
14. Clortran
15. Dentalone
16. Khloreton
17. Methaform
18. Sedaform
19. Chlorobutanol, Anhydrous
20. 2-propanol, 1,1,1-trichloro-2-methyl-
21. Trichlorisobutylalcohol
22. Trichloro-tert-butanol
23. Chlorobutanolum
24. Trichlorobutanol
25. Trichloro-t-butyl Alcohol
26. 2-(trichloromethyl)-2-propanol
27. 1,1,1-trichloro-tert-butyl Alcohol
28. Tert-trichlorobutyl Alcohol
29. Beta,beta,beta-trichloro-tert-butyl Alcohol
30. Trichloro-tert-butyl Alcohol
31. Nsc 44794
32. Chlorobutanol Hemihydrate
33. 2-(trichloromethyl)propan-2-ol
34. 2-propanol, 2-methyl-1,1,1-trichloro-
35. Hm4yqm8wrc
36. Nsc-44794
37. 1,1,1-trichloro-2-methyl-propan-2-ol
38. Mfcd00004461
39. 6001-64-5
40. Ncgc00159392-02
41. Ncgc00159392-05
42. Chlorbutanolum
43. Chlorbutolum
44. Chlorobutanol Hydrate
45. Dsstox_cid_21217
46. Dsstox_rid_79651
47. Wln: Qx1&1&xggg
48. Dsstox_gsid_41217
49. .beta.,.beta.,.beta.-trichloro-tert-butyl Alcohol
50. Caswell No. 185
51. Clorobutanolo [dcit]
52. 1,1-trichloro-tert-butyl Alcohol
53. Clorobutanol
54. Clorobutanolo
55. 1,1-trichloro-2-methyl-2-propanol
56. 2-propanol,1,1-trichloro-2-methyl-
57. Cas-57-15-8
58. Clorobutanol [inn-spanish]
59. Chlorobutanolum [inn-latin]
60. .beta.,.beta.-trichloro-tert-butyl Alcohol
61. Trichloro-2-methylpropan-2-ol
62. 28471-22-9
63. Hsdb 2761
64. Nsc-760101
65. Unii-hm4yqm8wrc
66. Einecs 200-317-6
67. Epa Pesticide Chemical Code 017501
68. Brn 0878167
69. 1, 1, 1-trichloro-2-methyl-2-propanol Hydrate
70. Acetonechloroform
71. Ai3-00048
72. 2-propanol, Trichloro-2-methyl-
73. Chloretone (tn)
74. Chlorobutanol [inn:ban:jan:nf]
75. Chloretone Hemihydrate
76. 2,2,2-trichloro-1,1-dimethylethanol
77. Chlorobutanol, Hydrous
78. T-trichlorobutyl Alcohol
79. Chlorobutanol [ii]
80. Chlorobutanol [mi]
81. Chlorobutanol [inn]
82. Chlorobutanol [jan]
83. Schembl1040
84. Chlorobutanol [hsdb]
85. Chlorobutanol [inci]
86. Acetone Chloroform Hemihydrate
87. Chlorobutanol [vandf]
88. 4-01-00-01629 (beilstein Handbook Reference)
89. 2-trichhloromethyl-2-propanol
90. Chlorobutanol [usp-rs]
91. Chlorobutanol [who-dd]
92. Chlorobutanol [who-ip]
93. Chembl1439973
94. Chlorobutanol (jp17/nf/inn)
95. Dtxsid1041217
96. 2-(trichloromethyl)-propan-2-ol
97. Nsc4596
98. Nsc5208
99. Chebi:134813
100. Molport-003-925-931
101. Chlorobutanol [green Book]
102. Hms3264c17
103. Pharmakon1600-01506102
104. Cs-b1703
105. Hy-b1263
106. Nsc-4596
107. Nsc-5208
108. Nsc44794
109. Zinc1482005
110. Chlorobutanol [ep Monograph]
111. Tox21_111629
112. Bdbm50417941
113. Nsc760101
114. S3705
115. Chlorobutanol, Anhydrous [ii]
116. Akos003619059
117. Chlorobutanolum [who-ip Latin]
118. Tox21_111629_1
119. 2-methyl-1,1,1-trichloro-2-propanol
120. Ccg-213842
121. Db11386
122. Ncgc00159392-03
123. Ncgc00159392-04
124. Chlorobutanol, Anhydrous [who-ip]
125. Cs-15316
126. Sy277495
127. Ft-0605936
128. Ft-0612881
129. T0386
130. Chlorobutanol, Anhydrous [ep Impurity]
131. En300-19331
132. C13278
133. D01942
134. Ab01563200_01
135. 1,1,1-trichloro-2-methylpropan-2-ol Hemihydrate
136. Sr-01000944257
137. Q1047468
138. Sr-01000944257-1
139. W-105484
140. Z1259084902
| Molecular Weight | 177.45 g/mol |
|---|---|
| Molecular Formula | C4H7Cl3O |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 175.956248 g/mol |
| Monoisotopic Mass | 175.956248 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 83.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic alpha-Agonists; Adrenergic Agents; Sympathomimetics; Vasoconstrictor Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
TOPICALLY AS SOLN IN CLOVE OIL AS DENTAL ANALGESIC. IT HAS LOCAL ANESTHETIC POTENCY TO MILD DEGREE & HAS BEEN EMPLOYED AS ANESTHETIC DUSTING POWDER (1 TO 5%) OR OINTMENT (10%).
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1222
WHEN ADMIN ORALLY, IT HAS MUCH THE SAME THERAPEUTIC USE AS CHLORAL HYDRATE. HENCE, CHLOROBUTANOL HAS BEEN EMPLOYED AS SEDATIVE & HYPNOTIC. IT HAS BEEN TAKEN ORALLY TO ALLAY VOMITING DUE TO GASTRITIS. DOSE--TOPICAL. ... IN TABLETS OR CAPSULES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1222
MEDICATION (VET): ANTISEPTIC & LOCAL ANESTHETIC; INTERNALLY, IT IS USED AS SEDATIVE & HYPNOTIC. IT APPEARS TO BE OF VALUE IN GASTRITIS WITH PERSISTENT VOMITING IN DOGS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1222
For more Therapeutic Uses (Complete) data for CHLORETONE (7 total), please visit the HSDB record page.
VET WARNING: ... NOT FOR USE AS MOTION SICKNESS DRUG IN CATS AS REPEATED USE IN THIS SPECIES CAUSES RESP CENTER DEPRESSION & MAY BE FATAL. AVOID USE IN ANIMALS WITH LIVER OR KIDNEY PATHOLOGY.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 104
RESEMBLES CHLORAL HYDRATE BUT NO GASTRIC IRRITATION.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-177
ALLERGIC REACTIONS ... INCLUDE ERYTHEMA, SCARLATINIFORM EXANTHEMS, URTICARIA, AND ECZEMATOID DERMATITIS. THE ERUPTION USUALLY BEGINS ON THE FACE OR BACK AND SPREADS TO THE NECK, CHEST, AND ARMS; IT MAY BE FOLLOWED BY DESQUAMATION ... /CHLORAL HYDRATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 128
UNDESIRABLE CNS EFFECTS INCLUDE LIGHTHEADEDNESS, MALAISE, ATAXIA, & NIGHTMARES. "HANGOVER" ALSO MAY OCCUR ... /CHLORAL HYDRATE/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 381
4. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG, BETWEEN 1 TEASPOON & 1 OZ FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-177
No approved therapeutic indications on its own.
Chlorobutanol is a detergent preservative with a broad spectrum of antimicrobial activity. _In vitro_, chlorobutanol demonstrated to inhibit platelet aggregation and release via unknown mechanisms. A study proposes that the antiplatelet effect of chlorobutanol may occur from inhibition of the arachidonic acid pathway. It attenuated thromboxane B2 formation, elevation of cytosolic free calcium, and ATP release, and additionally exhibited a significant inhibitory activity toward several aggregation inducers in a time- and concentration-dependent manner. Chlorobutanol may exert a direct negative inotropic effect on myocardial cells to isometric tension produced by the heart. Chlorobutanol was shown to induce conjunctival and corneal cell toxicity _in vitro_: at a concentration of 0.1%, Cbl caused near depletion of the squamous layer while degeneration of corneal epithelial cells, generation of conspicuous membranous blebs, cytoplasmic swelling, and occasional breaks in the external cell membrane were observed at a concentration of 0.5%.
Preservatives, Pharmaceutical
Substances added to pharmaceutical preparations to protect them from chemical change or microbial action. They include ANTI-BACTERIAL AGENTS and antioxidants. (See all compounds classified as Preservatives, Pharmaceutical.)
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AD - Other antiemetics
A04AD04 - Chlorobutanol
Absorption
Following oral administration in healthy subjects, the plasma concentration fell by 50% in 24 hours post-administration.
Route of Elimination
Under physiological conditions, chlorobutanol is unstable. The mean urinary recovery accounts for 9.6% of the dose orally administered.
Volume of Distribution
The volume of distribution was approximately 233 141 L in healthy individuals receiving oral chlorobutanol.
Clearance
In healthy subjects, the clearance was approximately 11.6 1.0 mL/min following oral administration.
Chlorobutanol is reported to undergo glucuronidation and sulphation.
Following oral administration, the terminal elimination half life in healthy subjects was 10.3 1.3 days.
As a detergent, chlorobutanol disrupts the lipid structure of the cell membrane and increases the cell permeability, leading to cell lysis. It induces conjunctival and corneal cell toxicity via causing cell retraction and cessation of normal cytokines, cell movement, and mitotic activity. It disrupts the barrier and transport properties of the corneal epithelium as well as inhibits the utilization of oxygen by the cornea. Chlorobutanol also inhibits oxygen use by the cornea, which increases susceptibility to infection.