
1. 569-57-3
2. Tace
3. Chlortrianizen
4. Chlortrianisestrol
5. Chlorotrianisine
6. Chlorestrolo
7. Chlorotrianizen
8. Chlortrianisen
9. Chloortrianisestrol
10. Clorotrisin
11. Hormonisene
12. Khlortrianizen
13. Clorestrolo
14. Merbentul
15. Anisene
16. Metace
17. Rianil
18. Chlortrianisoestrolum
19. Chlortrianisene
20. Chlorotrisin
21. Tri-p-anisylchloroethylene
22. Chlortrianisenum
23. Tace-fn
24. Tris(p-methoxyphenyl)chloroethylene
25. Clorotrianiseno
26. Trianisestrol
27. Chlorotrianisenum [inn-latin]
28. Chlorotrianisenum
29. Tace (pharmaceutical)
30. Chlorotris(p-methoxyphenyl)ethylene
31. Clorotrianiseno [inn-spanish]
32. Nsc-10108
33. Ethylene, Chlorotris(p-methoxyphenyl)-
34. Cta
35. 1-[1-chloro-2,2-bis(4-methoxyphenyl)ethenyl]-4-methoxybenzene
36. 1,1',1''-(1-chloro-1-ethenyl-2-ylidene)-tris(4-methoxybenzene)
37. Benzene, 1,1',1''-(1-chloro-1-ethenyl-2-ylidene)tris(4-methoxy)-
38. Chlorotrianisene (inn)
39. 4,4',4''-(2-chloroethene-1,1,2-triyl)tris(methoxybenzene)
40. Mls000028625
41. Chebi:3641
42. Clorotrianisene
43. Triagen
44. Ncgc00016511-02
45. Cas-569-57-3
46. Smr000058658
47. 1-[2-chloro-1,2-bis(4-methoxyphenyl)ethenyl]-4-methoxybenzene
48. 6v5034l121
49. 1,1',1''-(2-chloroethene-1,1,2-triyl)tris[4-(methyloxy)benzene]
50. Dsstox_cid_1299
51. Clorotrianisene [dcit]
52. Dsstox_rid_76067
53. Dsstox_gsid_21299
54. Chlorotrianisene [inn]
55. Benzene, 1,1',1''-(1-chloro-1-ethenyl-2-ylidene)tris(4-methoxy-
56. Benzene, 1,1',1''-(1-chloro-1-ethenyl-2-ylidene)tris[4-methoxy-
57. Ccris 4769
58. Hsdb 3302
59. Sr-01000721940
60. Einecs 209-318-6
61. Tace (tn)
62. Brn 1891845
63. Chlorotrianisene [nonsteroidal Oestrogens]
64. Chlorotrianisene [usp:inn:ban]
65. Prestwick_22
66. Unii-6v5034l121
67. Spectrum_000136
68. 1,1',1''-(2-chloroethene-1,1,2-triyl)tris(4-methoxybenzene)
69. Chlorotrianisene, ~95%
70. Opera_id_1728
71. Prestwick0_000757
72. Prestwick1_000757
73. Prestwick2_000757
74. Prestwick3_000757
75. Spectrum2_000704
76. Spectrum3_000343
77. Spectrum4_000954
78. Spectrum5_000711
79. Schembl8225
80. Bspbio_000774
81. Bspbio_002005
82. Chlorotrianisene [mi]
83. Kbiogr_001568
84. Kbioss_000596
85. 4-06-00-07650 (beilstein Handbook Reference)
86. Mls002415722
87. Spectrum1500181
88. Spbio_000887
89. Spbio_002713
90. Chlorotrianisene [hsdb]
91. Bpbio1_000852
92. Gtpl7146
93. Chlorotrianisene [vandf]
94. Chembl1200761
95. Chlorotrianisene [mart.]
96. Dtxsid1021299
97. Kbio2_000596
98. Kbio2_003164
99. Kbio2_005732
100. Kbio3_001225
101. Chlorotrianisene [who-dd]
102. Hms1570g16
103. Hms1920k17
104. Hms2091c20
105. Hms2097g16
106. Hms2230l03
107. Hms3371e11
108. Hms3714g16
109. Amy40000
110. Bcp13708
111. Hy-b2158
112. Nsc10108
113. Zinc1530598
114. Tox21_110466
115. Tox21_202361
116. Tox21_302897
117. Benzene, 1,1',1'-(1-chloro-1-ethenyl-2-ylidene)tris(4-methoxy)-
118. Ccg-40079
119. S4629
120. Chlorotrianisene [orange Book]
121. Wln: 1or Dyguyr Do1&r Do1
122. Akos015960863
123. Tox21_110466_1
124. Db00269
125. Ncgc00016511-01
126. Ncgc00016511-03
127. Ncgc00016511-04
128. Ncgc00016511-05
129. Ncgc00016511-06
130. Ncgc00016511-08
131. Ncgc00091333-01
132. Ncgc00091333-02
133. Ncgc00091333-03
134. Ncgc00256381-01
135. Ncgc00259910-01
136. Ac-12512
137. Bs-17089
138. Sbi-0051309.p003
139. Db-052990
140. Ab00051941
141. B5912
142. Cs-0020308
143. Ft-0632409
144. A16446
145. C75668
146. D00269
147. 569c573
148. A899783
149. Q5103213
150. Sr-01000721940-2
151. Sr-01000721940-3
152. 1,1',1''(1-chloro-1-ethenyl-2-ylidene)tris[4-methoxybenzene]
153. 1-[2-chloro-1,2-bis(4-methoxyphenyl)vinyl]-4-methoxybenzene #
154. Benzene,1',1''-(1-chloro-1-ethenyl-2-ylidene)tris[4-methoxy-
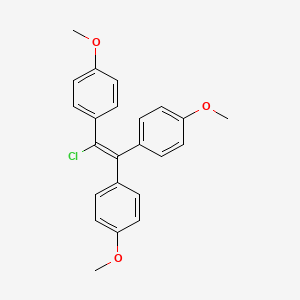
| Molecular Weight | 380.9 g/mol |
|---|---|
| Molecular Formula | C23H21ClO3 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 380.1179222 g/mol |
| Monoisotopic Mass | 380.1179222 g/mol |
| Topological Polar Surface Area | 27.7 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Hormonal; Estrogens, Non-Steroidal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...REPORTED RELIEF OF DYSMENORRHEA BY INHIBITING OVULATION WITH ESTROGEN. ... USE OF ESTROGENS IN TREATMENT OF ENDOMETRIOSIS... FAILURE OF OVARIAN DEVELOPMENT...IS TREATED WITH ESTROGEN... COMMON FORM OF ACNE IS FEATURE OF PUBERTY... TREATMENT WITH ESTROGEN IS EFFECTIVE... /ESTROGENS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1432
SENILE OR ATROPHIC VAGINITIS, OFTEN ASSOC WITH CHRONIC INFECTION OF ATROPHIC STRUCTURES, RESPONDS WELL TO ESTROGEN. KRAUROSIS VULVAE, DISTRESSINGLY ITCHY CONDITION DUE IN PART TO DEFICIENCY IN ESTROGEN...IS FAVORABLY INFLUENCED BY ESTROGEN SUPPLEMENTED BY LOCAL TREATMENT... /ESTROGENS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1432
...UNIQUE IN THAT ITS POTENCY IS GREATER BY ORAL THAN BY ANY OTHER ROUTE...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 918
For more Therapeutic Uses (Complete) data for CHLOROTRIANISENE (7 total), please visit the HSDB record page.
...LONG DURATION OF ACTION MAKES THIS DRUG UNSUITABLE FOR TREATMENT OF MENSTRUAL DISORDERS & OTHER CONDITIONS IN WHICH CYCLIC THERAPY IS DESIRED.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 918
...PREGNANT PT SHOULD NOT BE GIVEN ESTROGENS, PARTICULARLY DURING 1ST TRIMESTER-TIME WHEN FETAL REPRODUCTIVE TRACT IS DEVELOPING & MAY BE INFLUENCED BY EXOGENOUS ESTROGENS. /ESTROGENS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1429
...IS RELATED TO TRIPARANOL & TO CLOMIPHENE, BOTH OF WHICH CAN PRODUCE CATARACTS IN CERTAIN CIRCUMSTANCES. THIS SEEMS TO BE REASON FOR WATCHING FOR CATARACTS WHEN USING CHLOROTRIANISENE, BUT SO FAR THIS DRUG HAS NOT BEEN DEMONSTRATED TO PRODUCE THEM.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 281
Used to treat symptoms of menopause, deficiencies in ovary function (including underdevelopment of female sexual characteristics and some types of infertility), and in rare cases, prostate cancer. Chlorotrianisene may also be used to prevent breast engorgement following childbirth.
Chlorotrianisene is a nonsteroidal synthetic estrogen. After menopause, when the body no longer produces estrogen, chlorotrianisene is used as a simple replacement of estrogen. The estrogen-stimulated endometrium may bleed within 48-72 hours after discontinuance of estrogen therapy. Paradoxically, prolonged estrogen therapy may cause shrinkage of the endometrium and an increase in size of the myometrium. Estrogens have a weak anabolic effect and may cause sodium retention with associated fluid retention and edema. Estrogens may also decrease elevated blood cholesterol and phospholipid concentrations. Estrogens affect bone by increasing calcium deposition and accelerating epiphyseal closure, following initial growth stimulation. During the preovulatory or nonovulatory phase of the menstrual cycle, estrogen is the principal determinant in the onset of menstruation. A decline of estrogenic activity at the end of the menstrual cycle also may induce menstruation; however, the cessation of progesterone secretion is the most important factor during the mature ovulatory phase of the menstrual cycle. The benefit derived from estrogen therapy in the prevention of postpartum breast engorgement must be carefully weighed against the potential increased risk of puerperal thromboembolism associated with the use of large doses of estrogens.
Estrogens, Non-Steroidal
Non-steroidal compounds with estrogenic activity. (See all compounds classified as Estrogens, Non-Steroidal.)
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03C - Estrogens
G03CA - Natural and semisynthetic estrogens, plain
G03CA06 - Chlorotrianisene
Absorption
Absorption following oral administration is rapid.
...LONG-ACTING...BECAUSE OF SEQUESTRATION IN ADIPOSE TISSUE &, THEREFORE, IS NOT WIDELY USED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1431
...SUGGESTS THAT DRUG IS CONVERTED IN LIVER TO MORE ACTIVE FORM. ... IT IS STORED IN FAT, FROM WHICH IT IS SLOWLY RELEASED TO GIVE SUSTAINED ACTION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 918
ESTROGENIC ACTIVITY HAS BEEN FOUND IN ADIPOSE TISSUE UP TO MONTH AFTER CESSATION OF THERAPY.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 562
Metabolized principally in the liver, although the kidneys, gonads, and muscle tissues may be involved to some extent. The metabolic fate of the synthetic estrogens has not been fully elucidated.
RATE OF O-DEMETHYLATION WAS MAXIMAL AT 0.4 MMOLAR NADPH. ALTHOUGH NADH DID NOT CATALYZE REACTION ALONE, IT HAD SYNERGISTIC EFFECT IN PRESENCE OF EQUIMOLAR AMT OF NADPH. EXTRACTS FROM INCUBATION MIXT CONTAINED 1 MAJOR (MONO-O-DEMETHYLATED) & A MINOR (BIS-O-DEMETHYLATED) METAB.
RUENITZ PC; DRUG METAB DISPOS 6(6) 631 (1978)
Chlorotrianisene binds to the estrogen receptor on various estrogen receptor bearing cells. Target cells include cells in the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary.