


1. Chloroxine Hydrofluoride
2. Chlorquinol
3. Dichlorohydroxyquinoline
4. Dichloroquine
5. Quixalin
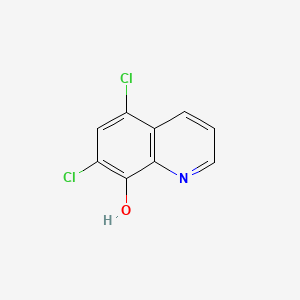
1. 773-76-2
2. 5,7-dichloroquinolin-8-ol
3. 5,7-dichloro-8-hydroxyquinoline
4. 5,7-dichloro-8-quinolinol
5. Capitrol
6. Chlorquinol
7. Dichloroxin
8. Quixalin
9. Chloroxyquinoline
10. Dichloroquinolinol
11. Dikhloroskin
12. Clofuzid
13. Endiaron
14. Quinolor
15. Dichlorohydroxyquinoline
16. Chlofucid
17. Quesyl
18. 5,7-dichlorooxine
19. 5,7-dichloroxine
20. 8-quinolinol, 5,7-dichloro-
21. 5,7-dichloro-8-oxyquinoline
22. Chlorohydroxyquinoline
23. Chloroxine [usan]
24. 5,7-dichlor-8-hydroxychinolin
25. Nsc 3904
26. Nsc-3904
27. Mfcd00006786
28. 2i8bd50i8b
29. 5,7-dichloro-8-hydroquinoline
30. Chebi:59477
31. Nsc3904
32. Chloroxine (usan)
33. As-229
34. Ncgc00095264-01
35. Dsstox_cid_2801
36. Dsstox_rid_76734
37. Dsstox_gsid_22801
38. Cas-773-76-2
39. Ccris 5751
40. Capitrol Cream Shampoo
41. Sr-01000747474
42. Einecs 212-258-3
43. Brn 0153606
44. Unii-2i8bd50i8b
45. 5,7-dichlor-8-hydroxychinolin [german]
46. Sq 16401
47. Capitrol (tn)
48. Spectrum_001434
49. Chloroxine [mi]
50. 5,7-dichloro-8-oxine
51. Spectrum2_000687
52. Spectrum3_001156
53. Spectrum4_000744
54. Spectrum5_001444
55. Chloroxine [vandf]
56. 8-quinolinol,7-dichloro-
57. Chloroxine [mart.]
58. Cid_2722
59. Schembl3350
60. Chloroxine [who-dd]
61. Oprea1_486275
62. Regid_for_cid_2722
63. Bspbio_002711
64. Kbiogr_001068
65. Kbioss_001914
66. 5-21-03-00286 (beilstein Handbook Reference)
67. Mls000681736
68. Bidd:gt0763
69. Divk1c_000578
70. Spectrum1503202
71. Spbio_000813
72. Zinc1131
73. Chembl1200596
74. Chloroxine [orange Book]
75. Dtxsid5022801
76. Bdbm32147
77. Hms501m20
78. Kbio1_000578
79. Kbio2_001914
80. Kbio2_004482
81. Kbio2_007050
82. Kbio3_002211
83. Ninds_000578
84. Hms1923k05
85. Hms2092n04
86. Hms2609a16
87. Hms3259e17
88. Hms3655g16
89. Hms3712o06
90. Pharmakon1600-01503202
91. Capitrol Cream Shampoo (salt/mix)
92. Amy23282
93. Component Of Capitrol Cream Shampoo
94. Hy-b0295
95. 5,7-dichloro-8-quinolinol, 99%
96. Tox21_111494
97. Ac9195
98. Ccg-40039
99. Nsc758398
100. S1839
101. Stk075368
102. 5,7-dichloroquinolin-8-ol;chloroxine
103. Akos000271338
104. Tox21_111494_1
105. Ac-4820
106. Db01243
107. Nc00505
108. Nsc-758398
109. Idi1_000578
110. Upcmld0enat5752182:001
111. Ncgc00095264-02
112. Ncgc00095264-03
113. Ncgc00095264-04
114. Ncgc00095264-05
115. Smr000312779
116. Sy016017
117. Tg2-36-1
118. Sbi-0051782.p002
119. A9795
120. D0412
121. Ft-0623709
122. Sw198618-2
123. D03472
124. Ab00052323-08
125. Ab00052323_09
126. Ab00052323_10
127. Sr-01000747474-2
128. Sr-01000747474-3
129. W-104320
130. Brd-k17075857-001-06-9
131. Q12029435
132. F0918-0943
| Molecular Weight | 214.04 g/mol |
|---|---|
| Molecular Formula | C9H5Cl2NO |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 212.9748192 g/mol |
| Monoisotopic Mass | 212.9748192 g/mol |
| Topological Polar Surface Area | 33.1 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 191 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of dandruff and mild to moderately severe seborrheic dermatitis of the scalp.
Chloroxine has an antibacterial action, inhibiting the growth of gram-positive as well as some gram-negative organisms. Also, chloroxine has shown some antifungal activity against certain dermatophytes and yeasts.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Although the mechanism of action is not understood, chloroxine may slow down mitotic activity in the epidermis, thereby reducing excessive scaling associated with dandruff or seborrheic dermatitis of the scalp. Chloroxine induces SOS-DNA repair in E. coli, so chloroxine may be genotoxic to bacteria.
