

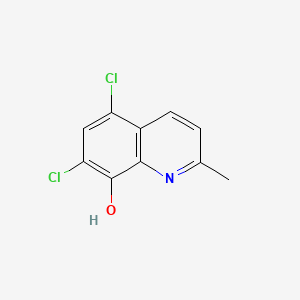

1. 5,7 Dichloro 2 Methyl 8 Quinolinol
2. 5,7-dichloro-2-methyl-8-quinolinol
3. Afungil
4. Chlorchinaldine
5. Chlorchinaldol
6. Chloroquinaldol
7. Sterosan
1. 72-80-0
2. 5,7-dichloro-8-hydroxyquinaldine
3. 5,7-dichloro-2-methylquinolin-8-ol
4. 5,7-dichloro-8-hydroxy-2-methylquinoline
5. Chloroquinaldol
6. 5,7-dichloro-2-methyl-8-quinolinol
7. Clorquinaldol
8. Sterosan
9. 5,7-dichloro-8-quinaldinol
10. Siasteran
11. Siosteran
12. Steroxin
13. Siogene
14. Chlorquinaldolum
15. Gyno-sterosan
16. 5,7-dichloro-2-methyl-8-hydroxyquinoline
17. 8-quinolinol, 5,7-dichloro-2-methyl-
18. Chloquinan
19. Hydroxydichloroquinaldine
20. Siogen
21. 5,7-dichloro-2-methyl-quinolin-8-ol
22. D6vhc87lls
23. Nsc-755830
24. Mls002695929
25. Chlorchinaldol
26. Chebi:74500
27. Chlorchinaldolum
28. Chlorquinaldol (inn)
29. Ncgc00095795-04
30. Clorchinaldolo
31. Smr001549973
32. 2-methyl-5,7-dichloro-8-hydroxyquinoline
33. Chlorquinaldol [inn]
34. Dsstox_cid_28924
35. Dsstox_rid_83190
36. Dsstox_gsid_48998
37. Chlorchinaldin
38. Chlorguinaldon
39. Clorchinaldolo [dcit]
40. Chlorquinaldol, Chlorquinaldol (5,7-dichloro-2-methyl-8-quinolinol)
41. Cas-72-80-0
42. Clorquinaldol [inn-spanish]
43. Chlorquinaldolum [inn-latin]
44. Chlorquinaldol [inn:ban:dcf]
45. Einecs 200-789-3
46. Unii-d6vhc87lls
47. Brn 0156683
48. Gynotherax
49. Vagisteran
50. Florabina
51. Saprosan
52. Siogenal
53. Siogeno
54. Siogenon
55. Siosept
56. Sterozan
57. Quesil
58. Mfcd00023984
59. Hydroxydichloroquinaldinol
60. Spectrum2_000524
61. Spectrum3_001092
62. Spectrum4_001263
63. Chlorquinaldol [mi]
64. Cid_6301
65. Oprea1_721210
66. Bspbio_002764
67. Kbiogr_001846
68. Spectrum212151
69. 5-21-03-00346 (beilstein Handbook Reference)
70. Schembl301405
71. Spbio_000507
72. Chlorquinaldol [mart.]
73. Chembl224325
74. Chlorquinaldol [who-dd]
75. Dtxsid3048998
76. Bdbm76302
77. Kbio3_001984
78. 5-bromo 2-isobutoxy Benzonitirle
79. Hms3089a18
80. Hms3264i07
81. Hms3652h09
82. Pharmakon1600-00212151
83. Zinc119403
84. Bcp11865
85. Hy-b1360
86. Tox21_113490
87. Ccg-39580
88. Nsc755830
89. S4192
90. Stl502989
91. Akos000119838
92. Tox21_113490_1
93. Cs-4899
94. Db13306
95. Nsc 755830
96. Ps-7753
97. S10253
98. Ncgc00095795-01
99. Ncgc00095795-02
100. Ncgc00095795-03
101. Ncgc00095795-06
102. Ac-29743
103. Quinolin-8-ol, 5,7-dichloro-2-methyl-
104. Sbi-0207012.p001
105. Db-055679
106. 5,7-bis(chloranyl)-2-methyl-quinolin-8-ol
107. Ft-0623717
108. Sw220230-1
109. A16448
110. D07208
111. 5,7-dichloro-8-hydroxy-2-methylquinoline, 98%
112. Ab00443827_06
113. Ab00443827_07
114. A837624
115. Sr-01000872737
116. Q1645622
117. Sr-01000872737-1
118. W-104478
119. Z118257784
120. 5,7-dichloro-8-hydroxyquinaldine 5,7-dichloro-2-methyl-8-hydroxyquinoline
| Molecular Weight | 228.07 g/mol |
|---|---|
| Molecular Formula | C10H7Cl2NO |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 226.9904692 g/mol |
| Monoisotopic Mass | 226.9904692 g/mol |
| Topological Polar Surface Area | 33.1 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 214 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Chlorquinaldol was used historically as a topical antiseptic agent for skin infections. It maintains use in European countries as a combination vaginal tablet with promestriene for use in the treatment of vaginal infections.
Chlorquinaldol is bacteriocidal in both gram positive and gram negative bacteria. It is more effective in targeting gram positive bacteria, particularly staphylococci.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AH - Quinoline derivatives
D08AH02 - Chlorquinaldol
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AC - Quinoline derivatives
G01AC03 - Chlorquinaldol
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AA - Hydroxyquinoline derivatives
P01AA04 - Chlorquinaldol
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA11 - Chlorquinaldol
Absorption
There is a high degree of variability in the extent of absorption of topically applied chorquinaldol preparations. It is reported to be between 4.2 and 23.5% of the applied dose. This is quite low compared to orally administered preparations which displayed 67.6% absorption in the same study.
Route of Elimination
Chlorquinadol is primarily excreted in the urine as the sulfate form. About 2% is excreted as the parent drug.
98% of drug is converted to the sulfate form and renally excreted.
The mechanism by which Chlorquinaldol exerts it's bacteriocidal effect is unknown. 8-hydroxyquinolines are known to be bidentate chelators of several metal ions which act as critical enzyme cofactors. However, the addition of exogenous metal ions does not appear to alter the minimum inhibitory concentration for mycobacterium tuberculosis suggesting that the primary mechanism does not rely on chelation.
