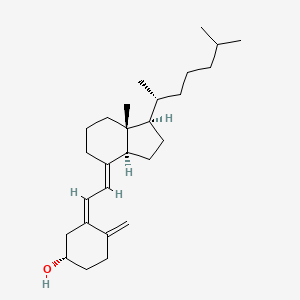

1. (3 Beta,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
2. Calciol
3. Cholecalciferols
4. Vitamin D 3
5. Vitamin D3
1. Vitamin D3
2. 67-97-0
3. Colecalciferol
4. Calciol
5. Oleovitamin D3
6. Ricketon
7. Arachitol
8. Trivitan
9. Deparal
10. Activated 7-dehydrocholesterol
11. Delsterol
12. Vigorsan
13. Ebivit
14. Vitamin D-3
15. Colecalcipherol
16. Quintox
17. Colecalciferolum
18. Cholecalciferolum
19. (+)-vitamin D3
20. D3-vicotrat
21. D3-vigantol
22. 1406-16-2
23. Vi-de-3-hydrosol
24. Neo Dohyfral D3
25. Vitinc Dan-dee-3
26. Cholecalciferol, D3
27. Vigantol
28. Vi-de3
29. Duphafral D3 1000
30. Feracol
31. Delta-d
32. Cc
33. Chebi:28940
34. (1s,3z)-3-[(2e)-2-[(1r,3as,7ar)-7a-methyl-1-[(2r)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
35. Nsc 375571
36. 9,10-secocholesta-5,7,10(19)-trien-3-beta-ol
37. Provitina
38. Micro-dee
39. 7-dehydrocholesterol, Activated
40. Vidde-3-hydrosol
41. Vitamin D3 Solution
42. Colecalciferol (inn)
43. Colecalciferol [inn]
44. Ncgc00159331-02
45. Rampage
46. Vitamin D3 10 Microg/ml In Acetonitrile
47. (3beta,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
48. (5z,7e)-(3s)-9,10-seco-5,7,10(19)-cholestatrien-3-ol
49. (5z,7e)-(3s)-9,10-secocholesta-5,7,10(19)-trien-3-ol
50. Dsstox_cid_6294
51. Dsstox_rid_78090
52. Dsstox_gsid_26294
53. Vitamin D3 (cholecalciferol)
54. 9,10-seco(5z,7e)-5,7,10(19)-cholestatrien-3beta-ol
55. Colecalciferolo
56. (3s,5z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
57. Colecalciferolo [dcit]
58. 9,10-secocholesta-5(z),7(e),10(19)-trien-3(.beta.)-ol
59. Vigantol Oil
60. (5e)-cholecalciferol
61. Nsc-375571
62. Colecalciferol D3
63. (1s,3z)-3-[(2e)-2-[(1r,3as,7ar)-1-[(1r)-1,5-dimethylhexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylene-cyclohexanol
64. (s,z)-3-(2-((1r,3as,7ar,e)-7a-methyl-1-((r)-6-methylheptan-2-yl)octahydro-4h-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol
65. Colecalciferolum [inn-latin]
66. Vitamin D 3
67. 7-dehydrocholestrol, Activated
68. Irradiated 7-dehydrocholesterol
69. Ccris 5813
70. Ccris 6286
71. Hsdb 820
72. Cholecalciferol Impurity A
73. 7-dehydrocholesterol, Irradiated
74. Vitamin D (cholecalciferol)
75. Vitamin D3 Emulsifiable
76. Einecs 200-673-2
77. Einecs 215-797-2
78. Mfcd00078131
79. Unii-9vu1ki44gp
80. Epa Pesticide Chemical Code 202901
81. Vitamin D3 (as Cholecalciferol)
82. Vitamin D3; Cholecalciferol
83. 1c6v77qf41
84. Devaron
85. Videkhol
86. Vitamin D Assay System Suitability
87. 7-dehydrocholesterol Activated
88. Unii-1c6v77qf41
89. Nsc375571
90. Granuvit D3
91. Dp-r206
92. Cas-67-97-0
93. Prestwick_63
94. Ak R215 Component Colecalciferol
95. Ak-r215 Component Colecalciferol
96. Cholecalciferol D3
97. Cyclohexanol, 3-((2e)-2-((1r,3as,7ar)-1-((1r)-1,5-dimethylhexyl)octahydro-7a-methyl-4h-inden-4-ylidene)ethylidene)-4-methylene-, (1s,3z)-
98. Cyclohexanol, 3-[(2e)-2-[(1r,3as,7ar)-1-[(1r)-1,5-dimethylhexyl]octahydro-7a-methyl-4h-inden-4-ylidene]ethylidene]-4-methylene-, (1s,3z)-
99. 9,10-secocholesta-5,7,10-trien-3-ol
100. Cholecalciferol [usp:ban:jan:iso]
101. ()-vitamin D3
102. 9,10-seco(5z,7e)-5,7,10(19)-cholestatrien-3-ol
103. Delta-d (tn)
104. 9,10-secocholesta-5,7,10(19)-trien-3-ol, (3beta,5z,7e)-
105. Prestwick3_000429
106. Bmse000507
107. Upcmld-dp152
108. Schembl3126
109. 9vu1ki44gp
110. Chembl1042
111. Bspbio_000418
112. Cholecalciferol; 67-97-0
113. Cholecalciferol (jp17/usp)
114. Bpbio1_000460
115. Megxm0_000458
116. Dtxsid6026294
117. Upcmld-dp152:001
118. Acon1_001997
119. Hms2096e20
120. Cholecalciferol, >=98% (hplc)
121. 9,10-secocholestra-5,7,10(19)-trien-3-ol, (3beta,5z,7e)-
122. Cholecalciferol, Analytical Standard
123. Zinc4474460
124. Tox21_111578
125. Tox21_202546
126. Bdbm50030475
127. Lmst03020001
128. S4063
129. Cholecalciferol For System Suitability
130. Akos015950641
131. Ac-8884
132. Ccg-268466
133. Cs-1179
134. Db00169
135. Smp1_000068
136. Vitamin D3 100 Microg/ml In Methanol
137. Ncgc00091072-01
138. Ncgc00159331-04
139. Ncgc00260095-01
140. Bs-42465
141. Hy-15398
142. Cholecalciferol (d3), Analytical Standard
143. C05443
144. D00188
145. 9,10-secocholesta-5,7,10(19)-trien-3-ol
146. Cholecalciferol, Meets Usp Testing Specifications
147. 078v131
148. 9,10-secocholesta-5,7,10(19)-trien-3?-ol
149. Q139347
150. (5e,7e)-9,10-secocholesta-5,7,10-trien-3-ol
151. Q-201931
152. 3-beta,z,7e-9,10-secocholestr-5,7,10(19)-trien-3-ol
153. Vitamin D3 Solution, 100 Mug/ml In Ethanol, 97% (cp)
154. (3beta,z,7e)-9,10-secocholesta-5,7,10(19)-trien-3-ol
155. 9,10-secocholesta-5,7,10(19)-trien-3-ol, (3b,5z,7e)-
156. Cholecalciferol, European Pharmacopoeia (ep) Reference Standard
157. Colecalciferol, British Pharmacopoeia (bp) Reference Standard
158. Cholecalciferol, United States Pharmacopeia (usp) Reference Standard
159. Cholecalciferol For System Suitability, European Pharmacopoeia (ep) Reference Standard
160. Vitamin D3 Solution, 1 Mg/ml In Ethanol, Ampule Of 1 Ml, Certified Reference Material
161. (1s,3z)-3-[(2e)-2-[7a-methyl-1-(6-methylheptan-2-yl)-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
162. Cholecalciferol (vitamin D3), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 384.6 g/mol |
|---|---|
| Molecular Formula | C27H44O |
| XLogP3 | 7.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 6 |
| Exact Mass | 384.339216023 g/mol |
| Monoisotopic Mass | 384.339216023 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 610 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Vitamin d |
| PubMed Health | Vitamin D |
| Drug Classes | Nutriceutical, Nutritive Agent, Vitamin Combination, Adult Formula |
| Active Ingredient | Ergocalciferol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50,000 iu |
| Market Status | Prescription |
| Company | Banner Pharmacaps |
| 2 of 2 | |
|---|---|
| Drug Name | Vitamin d |
| PubMed Health | Vitamin D |
| Drug Classes | Nutriceutical, Nutritive Agent, Vitamin Combination, Adult Formula |
| Active Ingredient | Ergocalciferol |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 50,000 iu |
| Market Status | Prescription |
| Company | Banner Pharmacaps |
Bone Density Conservation Agents; Vitamins
National Library of Medicine, SIS; ChemIDplus Record for Cholecalciferol (67-97-0), MESH Heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION (VET): Nutritional factor (Antirachitic)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1787
Therapeutic doses of specific vitamin D analogs are used in the treatment of chronic hypocalcemia, hypophosphatemia, rickets, and osteodystrophy associated with various medical conditions including chronic renal failure, familial hypophosphatemia, and hypoparathyroidism (postsurgical or idiopathic, or pseudohypoparathyroidism). Some analogs have been found to reduct elevated parathyroid hormone concentrations in patients with renal osteodystrophy associated with hyperparathyroidism. Theoretically, any of the vitamin D analogs may be used for the above conditions, However, because of their pharmacologic properties, some may be more useful in certain situations than others. Alfacalcidol, calcitriol, and dihydrotachysterol are usually preferred in patients with renal failure since these patients have impaired ability to synthesize calcitriol from cholecalciferol and ergocalciferol; therefore, the response is more predictable. In addition, their shorter half-lives may make toxicity easier to manage (hypercalcemia reverses more quickly). Ergocalciferol may not be the preferred agent in the treatment of familial hypophosphatemia or hypoparathyroidism because the large doses needed are associated with a risk of overdose and hypercalcemia; dihydrotachysterol and calcitriol may be preferred. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2966
Studies have shown that the elderly may have an increased need for vitamin D due to a possible decrease in the capacity of the skin to produce previtamin D3 or a decrease in exposure to the sun or impaired renal function or impaired vitamin D absorption.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Doses of vitamin D analogs that do not exceed the physiologic requirement are usually nontoxic. However, some infants and patients with sarcoidosis or hypoparathyroidism may have increased sensitivity to vitamin D analogs. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Acute or chronic administration of excessive doses of vitamin D analogs or enhanced responsiveness to physiologic amounts of ergocalciferol or cholecalciferol may lead to hypervitaminosis D manifested by hypercalcemia. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Decreased renal function without hypercalcemia has also been reported in patients with hypoparathyroidism after long-term vitamin D analog therapy. Before therapy with vitamin D analogs is initiated, serum phosphate concentrations must be controlled. To avoid ectopic calcification, the serum calcium (in mg/dL) times phosphorus (in mg/dL) should not be allowed to exceed 70. Because administration of vitamin D analogs may increase phosphate absorption, patients with renal failure may require adjustment in the dosage of aluminum-containing antacids used to decrease phosphate absorption. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
For more Drug Warnings (Complete) data for CHOLECALCIFEROL (10 total), please visit the HSDB record page.
Cholecalciferol use is indicated for the treatment of specific medical conditions like refractory rickets (or vitamin D resistant rickets), hypoparathyroidism, and familial hypophosphatemia. Concurrently, as one of the most commonly utilized forms of vitamin D, cholecalciferol is also very frequently used as a supplement in individuals to maintain sufficient vitamin d levels in the body or to treat vitamin D deficiency, as well as various medical conditions that can be associated directly or indirectly with vitamin d insufficiency like osteoporosis and chronic kidney disease, among others.
Treatment of osteoporosis
The in vivo synthesis of the predominant two biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of vitamin D3 cholecalciferol (or D2) occurs in the liver to yield 25-hydroxyvitamin D while the second hydroxylation happens in the kidneys to give 1, 25-dihydroxyvitamin D. These vitamin D metabolites subsequently facilitate the active absorption of calcium and phosphorus in the small intestine, serving to increase serum calcium and phosphate levels sufficiently to allow bone mineralization. Conversely, these vitamin D metabolites also assist in mobilizing calcium and phosphate from bone and likely increase the reabsorption of calcium and perhaps also of phosphate via the renal tubules. There exists a period of 10 to 24 hours between the administration of cholecalciferol and the initiation of its action in the body due to the necessity of synthesis of the active vitamin D metabolites in the liver and kidneys. It is parathyroid hormone that is responsible for the regulation of such metabolism at the level of the kidneys.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Calcium-Regulating Hormones and Agents
Hormones and molecules with calcium-regulating hormone-like actions that modulate OSTEOLYSIS and other extra-skeletal activities to maintain calcium homeostasis. (See all compounds classified as Calcium-Regulating Hormones and Agents.)
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CC - Vitamin d and analogues
A11CC05 - Colecalciferol
Absorption
Cholecalciferol is readily absorbed from the small intestine if fat absorption is normal. Moreover, bile is necessary for absorption as well. In particular, recent studies have determined aspects about the absorption of vitamin D, like the fact that a) the 25-hydroxyvitamin D metabolite of cholecalciferol is absorbed to a greater extent than the nonhydroxy form of cholecalciferol, b) the quantity of fat with which cholecalciferol is ingested does not appear to largely affect its bioavailability, and c) age does not apparently effect vitamin D cholecalciferol.
Route of Elimination
It has been observed that administered cholecalciferol and its metabolites are excreted primarily in the bile and feces.
Volume of Distribution
Studies have determined that the mean central volume of distribution of administered cholecalciferol supplementation in a group of 49 kidney transplant patients was approximately 237 L.
Clearance
Studies have determined that the mean clearance value of administered cholecalciferol supplementation in a group of 49 kidney transplant patients was approximately 2.5 L/day.
Readily absorbed from small intestine (proximal or distal); cholecalciferol may be absorbed more rapidly and completely than ergocalciferol.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Elimination: Biliary/renal. /Vitamin D and analogs/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Many vitamin D analogs are readily absorbed from the GI tract following oral administration if fat absorption is normal. The presence of bile is required for absorption of ergocalciferol and the extent of GI absorption may be decreased in patients with hepatic, biliary, or GI disease (e.g., Crohn's disease, Whipple's disease, sprue). Because vitamin D is fat soluble, it is incorporated into chylomicrons and absorbed via the lymphatic system; approximately 80% of ingested vitamin D appears to be absorbed systemically through this mechanism, principally in the small intestine. Although some evidence suggested that intestinal absorption of vitamin D may be decreased in geriatric adults, other evidence did not show clinically important age-related alterations in GI absorption of the vitamin in therapeutic doses. It currently is not known whether aging alters the GI absorption of physiologic amounts of vitamin D. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
After absorption, ergocalciferol and cholecalciferol enter the blood via chylomicrons of lymph and then associate mainly with a specific alpha-globulin (vitamin D-binding protein). The hydroxylated metabolites of ergocalciferol and cholecalciferol also circulate associated with the same alpha-globulin. 25-Hydroxylated ergocalciferol and cholecalciferol are stored in fat and muscles for prolonged periods. Once vitamin D enters systemic circulation from lymph via the thoracic duct or from skin, it accumulates in the liver within a few hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
For more Absorption, Distribution and Excretion (Complete) data for CHOLECALCIFEROL (7 total), please visit the HSDB record page.
Within the liver, cholecalciferol is hydroxylated to calcifediol (25-hydroxycholecalciferol) by the enzyme vitamin D-25-hydroxylase. At the kidney, calcifediol subsequently serves as a substrate for 1-alpha-hydroxylase, yielding calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D3.
Metabolic activation of cholecalciferol and ergocalciferol occurs in 2 steps, the first in the liver and the second in the kidneys. Metabolic activation of calcifediol occurs in the kidneys; dihydrotachysterol, alfacalcidol and doxercalciferol are activated in the liver.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Normal combined (ie, 25-hydroxyvitamin D) plasma concentrations of 25-hydroxycholecalciferol (calcifediol) and 25-hydroxyergocalciferol, which are the major circulating metabolites of cholecalciferol and ergocalciferol, have been reported to range from 8-80 ng/mL, depending on the assay used, and vary with exposure to UV light. A commonly reported range for the lower limit of normal is 8-15 ng/mL, depending on geographic location (eg, Southern California would be higher than Massachusetts).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
In the liver, ergocalciferol and cholecalciferol are converted in the mitochondria to their 25-hydroxy derivatives by the enzyme vitamin D 25-hydroxylase. Vitamin D 25-hydroxylase activity is regulated in the liver by concentrations of vitamin D and its metabolites; therefore, increases in the systemic circulation of the 25-hydroxy metabolites following exposure to sunlight or ingestion of vitamin D are relatively modest compared with cumulative production or intake of the vitamin. Serum concentrations of nonhydroxylated vitamin D are short-lived as a result of storage in fat or metabolism in the liver. In the kidneys, these metabolites are further hydroxylated at the 1 position by the enzyme vitamin D 1-hydroxylase to their active forms, 1,25-dihydroxycholecalciferol (calcitriol) and 1,25-dihydroxyergocalciferol. ... Activity of the vitamin D 1-hydroxylase enzyme requires molecular oxygen, magnesium ion, and malate and is regulated principally by PTH in response to serum concentrations of calcium and phosphate, and perhaps by circulating concentrations of 1,25-dihydroxyergocalciferol and 1,25-dihydroxycholecalciferol. Other hormones (ie, cortisol, estrogens, prolactin, and growth hormone) also may influence the metabolism of cholecalciferol and ergocalciferol.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3543
The hepatic enzyme system responsible for 25-hydroxylation of vitamin D /(vitamin D-25 hydroxylase)/ is associated with the microsomal and mitochondrial fractions of homogenates and requires NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) and molecular oxygen. ... The enzyme system /in kidney/ responsible for 1-hydroxylation of 25-OHD (25-hydroxycholecalciferol) /(25-OHD-1-alpha-hydroxylase)/ is associated with mitochondria in the proximal tubules. It is a mixed function oxidase and requires molecular oxygen and NADPH as cofactors. Cytochrome P450, a flavoprotein, and ferredoxin are components of the enzyme complex.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1727
At this time, there have been resources that document the half-life of cholecalciferol as being about 50 days while other sources have noted that the half-life of calcitriol (1,25-dihydroxyvitamin D3) is approximately 15 hours while that of calcidiol (25-hydroxyvitamin D3) is about 15 days. Moreover, it appears that the half-lives of any particular administration of vitamin d can vary due to variations in vitamin d binding protein concentrations and genotype in particular individuals.
The Vitamin /D/ disappears from plasma with a half-life of 19 to 25 hr but is stored in fat depots for prolonged periods. ... The 25-hydroxy derivative has a biological half-life of 19 days ... The plasma half-life of calcitriol /(1,25-dihydroxy-vitamin D)/ is estimated to be between 3 and 5 days in human beings ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1730
Most individuals naturally generate adequate amounts of vitamin D through ordinary dietary intake of vitamin D (in some foods like eggs, fish, and cheese) and natural photochemical conversion of the vitamin D3 precursor 7-dehydrocholesterol in the skin via exposure to sunlight. Conversely, vitamin D deficiency can often occur from a combination of insufficient exposure to sunlight, inadequate dietary intake of vitamin D, genetic defects with endogenous vitamin D receptor, or even severe liver or kidney disease. Such deficiency is known for resulting in conditions like rickets or osteomalacia, all of which reflect inadequate mineralization of bone, enhanced compensatory skeletal demineralization, resultant decreased calcium ion blood concentrations, and increases in the production and secretion of parathyroid hormone. Increases in parathyroid hormone stimulate the mobilization of skeletal calcium and the renal excretion of phosphorus. This enhanced mobilization of skeletal calcium leads towards porotic bone conditions. Ordinarily, while vitamin D3 is made naturally via photochemical processes in the skin, both itself and vitamin D2 can be found in various food and pharmaceutical sources as dietary supplements. The principal biological function of vitamin D is the maintenance of normal levels of serum calcium and phosphorus in the bloodstream by enhancing the efficacy of the small intestine to absorb these minerals from the diet. At the liver, vitamin D3 or D2 is hydroxylated to 25-hydroxyvitamin D and then finally to the primary active metabolite 1,25-dihydroxyvitamin D in the kidney via further hydroxylation. This final metabolite binds to endogenous vitamin d receptors, which results in a variety of regulatory roles - including maintaining calcium balance, the regulation of parathyroid hormone, the promotion of the renal reabsorption of calcium, increased intestinal absorption of calcium and phosphorus, and increased calcium and phosphorus mobilization of calcium and phosphorus from bone to plasma to maintain balanced levels of each in bone and the plasma. In particular, calcitriol interacts with vitamin D receptors in the small intestine to enhance the efficiency of intestinal calcium and phosphorous absorption from about 10-15% to 30-40% and 60% increased to 80%, respectively. Furthermore, calcitriol binds with vitamin D receptors in osteoblasts to stimulate a receptor activator of nuclear factor kB ligand (or RANKL) which subsequently interacts with receptor activator of nuclear factor kB (NFkB) on immature preosteoclasts, causing them to become mature bone-resorbing osteoclasts. Such mature osteoclasts ultimately function in removing calcium and phosphorus from bone to maintain blood calcium and phosphorus levels. Moreover, calcitriol also stimulates calcium reabsorption from the glomerular filtrate in the kidneys. Additionally, it is believed that when calcitriol binds with nuclear vitamin D receptors, that this bound complex itself binds to retinoic acid X receptor (RXR) to generate a heterodimeric complex that consequently binds to specific nucleotide sequences in the DNA called vitamin D response elements. When bound, various transcription factors attach to this complex, resulting in either up or down-regulation of the associated gene's activity. It is thought that there may be as much as 200 to 2000 genes that possess vitamin D response elements or that are influenced indirectly to control a multitude of genes across the genome. It is in this way that cholecalciferol is believed to function in regulating gene transcription associated with cancer risk, autoimmune disorders, and cardiovascular disease linked to vitamin D deficiency. In fact, there has been some research to suggest calcitriol may also be able to prevent malignancies by inducing cellular maturation and inducing apoptosis and inhibiting angiogenesis, exhibit anti-inflammatory effects by inhibiting foam cell formation and promoting angiogenesis in endothelial colony-forming cells in vitro, inhibit immune reactions by enhancing the transcription of endogenous antibiotics like cathelicidin and regulate the activity and differentiation of CD4+ T cells, amongst a variety of other proposed actions.
The principal biologic function of vitamin D is to maintain serum calcium and phosphorus concentrations within the normal range by enhancing the efficiency of the small intestine to absorb these minerals from the diet. Calcitriol (activated vitamin D) enhances the efficiency of intestinal calcium absorption along the entire small intestine, but principally in the duodenum and jejunum. Calcitriol also enhances phosphorus absorption along the entire small intestine, but principally in the jejunum and ileum. The activated forms of ergocalciferol, doxercalciferol, and cholecalciferol may have a negative feedback effect on parathyroid hormone (PTH) production.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3542
The most two important mechanisms by which vitamin D acts to maintain normal concentration of calcium and phosphate in plasma are to facilitate their absorption by the small intestine and to enhance their mobilization from bone. /Vitamin D/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1541