

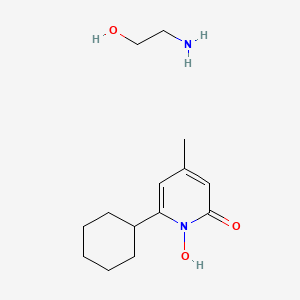

1. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Ethanolamine Salt
2. Batrafen
3. Ciclopirox
4. Ciclopiroxolamine
5. Cyclopirox
6. Cyclopyroxolamine
7. Dafnegin Csc
8. Dafnegin-csc
9. Hoe 296
10. Hoe-296
11. Hoe296
12. Loprox
13. Penlac
1. 41621-49-2
2. Ciclopirox Ethanolamine
3. Ciclopiroxolamine
4. Batrafen
5. Micoxolamina
6. Brumixol
7. Mycoster
8. Ciclopiroxolamin
9. Ciclopirox (olamine)
10. Hoe 296
11. Ciclochem
12. Dafnegin
13. Hoe-296
14. Ciclopirox Ethanolamine Salt (1:1)
15. 2-aminoethanol;6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one
16. Mfcd00078997
17. Nsc-336278
18. 50md4sb4ap
19. Ciclobirox Olamine
20. Mls003170863
21. Nsc336278
22. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Compound With 2-aminoethanol (1:1)
23. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one Compound With 2-aminoethanol (1:1)
24. Ncgc00017112-01
25. Cas-41621-49-2
26. Loprox (tn)
27. 2(1h)-pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-, Compound With 2-aminoethanol (1:1)
28. Dsstox_cid_25583
29. Dsstox_rid_80979
30. Dsstox_gsid_45583
31. Mls002153867
32. 2-aminoethan-1-ol; 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one
33. Sr-05000001589
34. Smr001233223
35. Einecs 255-464-9
36. Unii-50md4sb4ap
37. Nsc 336278
38. Kopcycloamine
39. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Ethanolamine Salt
40. Rv4104a
41. Penlac Nail Lacquer
42. Prestwick_785
43. Ciclopirox Olamine [usan:usp:jan]
44. Spectrum_000150
45. Ciclopirox Olamine,(s)
46. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone, 2-aminoethanol Salt
47. Ciclopirox Olamine- Bio-x
48. Ciclopirox Olamine Usp/ep
49. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridon, 2-aminoethanol-salz [german]
50. 2(1h)-pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-, Compd. With 2-aminoethanol (1:1)
51. 2-aminoethanol Compd. With 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone (1:1)
52. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone Compd. With 2-aminoethanol (1:1)
53. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one, Compound With 2-aminoethanol (1:1)
54. Schembl66960
55. Kbioss_000630
56. Divk1c_000705
57. Spectrum1500189
58. Ciclopirox Olamine (jan/usp)
59. Chembl242580
60. Ciclopirox Olamine [jan]
61. Dtxsid6045583
62. Hms502d07
63. Hy-b0450a
64. Kbio1_000705
65. Kbio2_000630
66. Kbio2_003198
67. Kbio2_005766
68. Ciclopirox Olamine [inci]
69. Ciclopirox Olamine [usan]
70. Ninds_000705
71. Ciclopirox Olamine [vandf]
72. Hms1569n03
73. Hms1920o09
74. Hms2091e16
75. Hms2096n03
76. Hms2232b06
77. Hms3656b16
78. Hms3713n03
79. Pharmakon1600-01500189
80. Ciclopirox Olamine [mart.]
81. Ciclopirox Olamine [usp-rs]
82. Ciclopirox Olamine [who-dd]
83. Tox21_110781
84. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridon, 2-aminoethanol-salz
85. Ccg-38945
86. Nsc756694
87. S3019
88. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one 2-aminoethanol (1:1)
89. Akos015891685
90. Tox21_110781_1
91. Ciclopirox Olamine, Analytical Standard
92. Cs-4767
93. Ks-5086
94. Nsc-756694
95. Ciclopirox Olamine [ep Impurity]
96. Idi1_000705
97. Ciclopirox Olamine [ep Monograph]
98. Ncgc00017112-02
99. Ncgc00017112-03
100. Ncgc00017112-10
101. Ncgc00094623-01
102. Ncgc00094623-02
103. Ac-14469
104. Bc164302
105. Ciclopirox Ethanolamine;ciclopirox Olamine
106. Ciclopirox Olamine [usp Monograph]
107. Nci60_002954
108. Sy102087
109. C3545
110. Ft-0602960
111. Sw196923-4
112. 42c050
113. D01364
114. A825610
115. Ciclopirox Ethanolamine Salt (1:1) [mi]
116. Sr-05000001589-1
117. Sr-05000001589-3
118. 2(1h)-pyridinone, Compd. With 2-aminoethanol (1:1)
119. 2-aminoethanol; 6-cyclohexyl-1-hydroxy-4-methyl-pyridin-2-one
120. Ciclopirox Olamine, European Pharmacopoeia (ep) Reference Standard
121. 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1h)-one 2-aminoethanol Salt
122. Ciclopirox Olamine, United States Pharmacopeia (usp) Reference Standard
123. 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone Compound With 2-aminoethanol
| Molecular Weight | 268.35 g/mol |
|---|---|
| Molecular Formula | C14H24N2O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 268.17869263 g/mol |
| Monoisotopic Mass | 268.17869263 g/mol |
| Topological Polar Surface Area | 86.8 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 335 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
