

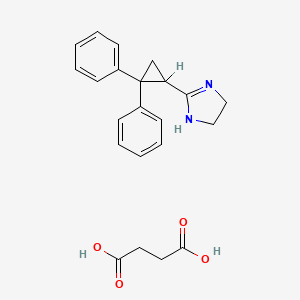

1. 2-(2,2-diphenylcyclopropyl)-2-imidazoline
2. Cibenzoline
3. Cifenline
4. Cifenline Succinate
5. Cifenline, (s)-isomer
6. Cipralan
7. Exacor
1. 100678-32-8
2. Cifenline Succinate
3. Cipralan
4. Cibenol
5. Cifenline Succinate [usan]
6. 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-1h-imidazole Succinate
7. 85589-37-3
8. Cibenzoline Succinate [jan]
9. Ro 22-7796/001
10. 38g16rwj37
11. Butanedioic Acid, Compd. With 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-1h-imidazole (1:1)
12. Cipralan; Exacor; Ro 22-7796/001
13. Ritmalan
14. Exacor
15. Butanedioic Acid;2-(2,2-diphenylcyclopropyl)-4,5-dihydro-1h-imidazole
16. Cifenline Succinate (usan)
17. Cibenzoline Succinate (1:1)
18. Ncgc00162222-01
19. Ro-227796001
20. Unii-38g16rwj37
21. Cipralan (tn)
22. Cibenol (tn)
23. (+-)-2-(2,2-diphenylcyclopropyl)-2-imidazoline Succinate (1:1)
24. 4,5-dihydro-2-(2,2-diphenylcyclopropyl)-1h-imidazole Succinate
25. 1h-imidazole, 4,5-dihydro-2-(2,2-diphenylcyclopropyl)-, Succinate
26. 1h-imidazole, 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-, (+-)-, Butanedioate (1:1)
27. Butanedioic Acid, Compd. With 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-1h-imidazole
28. Dsstox_cid_27797
29. Dsstox_rid_82569
30. Dsstox_gsid_47820
31. Schembl121044
32. Cibenzoline Succinate (jp17)
33. Chembl3189111
34. Cifenline Succinate [mi]
35. Dtxsid0047820
36. Chebi:31396
37. Hms3262i07
38. Tox21_112004
39. Tox21_500683
40. Akos016339647
41. Cibenzoline Succinate [who-dd]
42. Ccg-221987
43. Ks-1343
44. Lp00683
45. Ro-227796
46. Ncgc00261368-01
47. Up-33901
48. Cas-100678-32-8
49. Ft-0623817
50. Cibenzoline Succinate, >=97% (nmr), Solid
51. D01455
52. 678c328
53. Ro-22-7796/001
54. J-000196
55. Q27256782
56. 1-[2-(z)-methoxyimino-2-(2-aminothiazol-4-yl)acetoxy]benzotrizole
57. (+/-)-2-(2,2-diphenylcyclopropyl)-2-imidazoline Succinate (1:1)
58. 1h-imidazole, 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-, (+/-)-, Butanedioate (1:1)
| Molecular Weight | 380.4 g/mol |
|---|---|
| Molecular Formula | C22H24N2O4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 380.17360725 g/mol |
| Monoisotopic Mass | 380.17360725 g/mol |
| Topological Polar Surface Area | 99 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)