


1. Cilastatin
2. Cilastatin Monosodium Salt
3. Mk 0791
4. Mk 791
5. Mk-791
6. Mk0791
7. Mk791
8. Monosodium Salt, Cilastatin
9. Salt, Cilastatin Monosodium
10. Sodium, Cilastatin
1. 81129-83-1
2. Cilastatin Sodium Salt
3. Cilastatin Na
4. Mls001401364
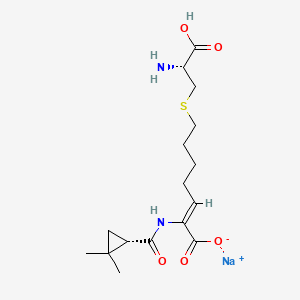
5. Sodium;(z)-7-[(2r)-2-amino-2-carboxyethyl]sulfanyl-2-[[(1s)-2,2-dimethylcyclopropanecarbonyl]amino]hept-2-enoate
6. Mk-791
7. Dsstox_cid_26915
8. Dsstox_rid_82013
9. Dsstox_gsid_46915
10. Sodium;(z)-7-[(2s)-2-amino-2-carboxyethyl]sulfanyl-2-[[(1s)-2,2-dimethylcyclopropanecarbonyl]amino]hept-2-enoate
11. Chebi:59511
12. Cas-81129-83-1
13. Ncgc00181346-01
14. Cilastatino
15. Cilastatin Sodium, Bio-x
16. Ncgc00167838-01
17. Chembl1201057
18. Cilastatin Sodium (jp17/usp)
19. Dtxsid2046915
20. Hms2051l07
21. Hms2233j15
22. Ex-a4989
23. Tox21_112591
24. Tox21_112804
25. Mfcd08459332
26. Akos015962007
27. Ac-3375
28. Ccg-100932
29. Nc00182
30. Bc164306
31. Smr000469147
32. Sodium (z)-7-(((r)-2-amino-2-carboxyethyl)thio)-2-((s)-2,2-dimethylcyclopropane-1-carboxamido)hept-2-enoate
33. Cilastatin Sodium 100 Microg/ml In Water
34. B7156
35. D02194
36. L-642957
37. [r-[r*,s*-(z)]]-7-[(2-amino-2-carboxyethyl)thio]-2- [[(2,2-dimethylcyclopropyl)carbonyl]amino]-2- Heptenoic Acid Monosodium Salt
38. Sodium(z)-7-(((r)-2-amino-2-carboxyethyl)thio)-2-((s)-2,2-dimethylcyclopropane-1-carboxamido)hept-2-enoate
| Molecular Weight | 380.4 g/mol |
|---|---|
| Molecular Formula | C16H25N2NaO5S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 380.13818736 g/mol |
| Monoisotopic Mass | 380.13818736 g/mol |
| Topological Polar Surface Area | 158 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 525 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)