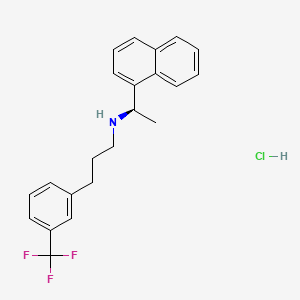

1. Alpha-methyl-n-(3-(3-(trifluoromethyl)phenyl)propyl)-1-naphthalenemethanamine, (alphar)-hydrochloride
2. Amg 073
3. Amg073
4. Cinacalcet
5. Krn 1493
6. Sensipar
1. 364782-34-3
2. Cinacalcet Hcl
3. Sensipar
4. Mimpara
5. Amg073 Hcl
6. Regpara
7. (r)-n-(1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine Hydrochloride
8. Cinacalcet, Hcl
9. Cinacalcet Hydrochloride [usan]
10. Cinacalcet (hydrochloride)
11. Amg-073 Hcl
12. Amg073 Hydrochloride
13. Chebi:48391
14. 1k860wsg25
15. Parareg
16. N-[(1r)-1-naphthalen-1-ylethyl]-3-[3-(trifluoromethyl)phenyl]propan-1-amine;hydrochloride
17. Amg-073 Hcl (cinacalcet Hydrochloride)
18. 364782-34-3 (hcl)
19. [(1r)-1-(naphthalen-1-yl)ethyl]({3-[3-(trifluoromethyl)phenyl]propyl})amine Hydrochloride
20. Dsstox_cid_26792
21. Dsstox_rid_81909
22. N-((1r)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine Hydrochloride
23. N-[(1r)-1-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]propan-1-amine Hydrochloride
24. Dsstox_gsid_46792
25. (r)-n-(1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoro-methyl)phenyl)propan-1-amine Hydrochloride
26. Smr002530058
27. Cas-364782-34-3
28. Krn 1493
29. Ncgc00181002-01
30. Amg 073 Hcl
31. Unii-1k860wsg25
32. Sensipar (tn)
33. Mimpara (tn)
34. Cnc-hcl
35. Cinacelcet Hydrochloride
36. Sensipar(tm)
37. Amg-073 Hydrochloride
38. Amg-073.hcl
39. Mls004774045
40. Mls006010213
41. Chembl1200776
42. Dtxsid3046792
43. Bcpp000409
44. Bcp02533
45. Nps-1493
46. Tox21_112654
47. Cinacalcet Hydrochloride (jan/usan)
48. Cinacalcet Hydrochloride [mi]
49. Hy-70037a
50. Mfcd08067750
51. S1260
52. Cinacalcet Hydrochloride [jan]
53. Akos005146514
54. Akos015969126
55. Tox21_112654_1
56. Ac-1799
57. Am90312
58. Bcp9000286
59. Ccg-268579
60. Cs-0288
61. Gs-4171
62. Cinacalcet Hydrochloride [mart.]
63. Cinacalcet Hydrochloride [usp-rs]
64. Cinacalcet Hydrochloride [who-dd]
65. Ncgc00181002-04
66. 1-naphthalenemethanamine, Alpha-methyl-n-(3-(3-(trifluoromethyl)phenyl)propyl)-, (alphar)-, Hydrochloride
67. Sw219246-1
68. Cinacalcet Hydrochloride [orange Book]
69. D03505
70. 782c343
71. J-520045
72. Q27121179
73. Z1741977003
74. (r)-n-(1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine Hcl
75. (r)-n-(3-(3-(trifluoromethyl)phenyl)propyl)-1- -(1-napthyl)ethylamine Hydrochloride
76. (alphar)-alpha-methyl-n-[3-[3-(trifluoromethyl)phenyl)propyl]-1-napthalenemethanamine Hydrochloride
77. 1-naphthalenemethanamine, .alpha.-methyl-n-(3-(3-(trifluoromethyl)phenyl)propyl)-, (.alpha.r)-, Hydrochloride
78. 1-naphthalenemethanamine, Alpha-methyl-n-[3-[3-(trifluoromethyl)phenyl]propyl]-, Hydrochloride (1:1), (alphar)-
79. 1-naphthalenemethanamine,a-methyl-n-[3-[3-(trifluoromethyl)phenyl]propyl]-, Hydrochloride, (ar)-
| Molecular Weight | 393.9 g/mol |
|---|---|
| Molecular Formula | C22H23ClF3N |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 393.1471119 g/mol |
| Monoisotopic Mass | 393.1471119 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 422 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Cinacalcet hydrochloride |
| PubMed Health | Cinacalcet (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | Sensipar (cinacalcet) is a calcimimetic agent that increases the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. Its empirical formula is C22H22F3NHCl with a molecular weight of 393.9 g/mol (hydrochloride salt... |
| Active Ingredient | Cinacalcet hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 60mg; 30mg; 90mg |
| Market Status | Tentative Approval |
| Company | Teva Pharms; Barr |
| 2 of 4 | |
|---|---|
| Drug Name | Sensipar |
| Drug Label | Sensipar (cinacalcet) is a calcimimetic agent that increases the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. Its empirical formula is C22H22F3NHCl with a molecular weight of 393.9 g/mol (hydrochloride salt... |
| Active Ingredient | Cinacalcet hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 90mg base; eq 30mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Amgen |
| 3 of 4 | |
|---|---|
| Drug Name | Cinacalcet hydrochloride |
| PubMed Health | Cinacalcet (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | Sensipar (cinacalcet) is a calcimimetic agent that increases the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. Its empirical formula is C22H22F3NHCl with a molecular weight of 393.9 g/mol (hydrochloride salt... |
| Active Ingredient | Cinacalcet hydrochloride |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 60mg; 30mg; 90mg |
| Market Status | Tentative Approval |
| Company | Teva Pharms; Barr |
| 4 of 4 | |
|---|---|
| Drug Name | Sensipar |
| Drug Label | Sensipar (cinacalcet) is a calcimimetic agent that increases the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. Its empirical formula is C22H22F3NHCl with a molecular weight of 393.9 g/mol (hydrochloride salt... |
| Active Ingredient | Cinacalcet hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 90mg base; eq 30mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Amgen |
* Secondary hyperparathyroidism :
Adults
Treatment of secondary hyperparathyroidism (HPT) in adult patients with end-stage renal disease (ESRD) on maintenance dialysis therapy.
Paediatric population
Treatment of secondary hyperparathyroidism (HPT) in children aged 3 years and older with end-stage renal disease (ESRD) on maintenance dialysis therapy in whom secondary HPT is not adequately controlled with standard of care therapy (see section 4. 4).
Cinacalcet Accordpharma may be used as part of a therapeutic regimen including phosphate binders and/or Vitamin D sterols, as appropriate (see section 5. 1).
* Parathyroid carcinoma and primary hyperparathyroidism in adults:
Reduction of hypercalcaemia in adult patients with:
- parathyroid carcinoma.
- primary HPT for whom parathyroidectomy would be indicated on the basis of serum calcium levels (as defined by relevant treatment guidelines), but in whom parathyroidectomy is not clinically appropriate or is contraindicated.
Treatment of secondary hyperparathyroidism (HPT) in patients with end-stage renal disease (ESRD) on maintenance dialysis therapy.
Cinacalcet Mylan may be used as part of a therapeutic regimen including phosphate binders and/or vitamin D sterols, as appropriate.
Reduction of hypercalcaemia in patients with:
- parathyroid carcinoma
- primary HPT for whom parathyroidectomy
would be indicated on the basis of serum calcium levels (as defined by relevant treatment guidelines), but in whom parathyroidectomy is not clinically appropriate or is contraindicated.
* Secondary hyperparathyroidism :
Adults
Treatment of secondary hyperparathyroidism (HPT) in adult patients with end stage renal disease (ESRD) on maintenance dialysis therapy.
Paediatric population
Treatment of secondary hyperparathyroidism (HPT) in children aged 3 years and older with end stage renal disease (ESRD) on maintenance dialysis therapy in whom secondary HPT is not adequately controlled with standard of care therapy.
Mimpara may be used as part of a therapeutic regimen including phosphate binders and/or Vitamin D sterols, as appropriate.
Parathyroid carcinoma and primary hyperparathyroidism in adults.
Reduction of hypercalcaemia in adult patients with:
- parathyroid carcinoma;
- primary HPT for whom parathyroidectomy would be indicated on the basis of serum calcium levels (as defined by relevant treatment guidelines), but in whom parathyroidectomy is not clinically appropriate or is contraindicated.
Treatment of secondary hyperparathyroidism (HPT) in patients with end-stage renal disease (ESRD) on maintenance dialysis therapy.
Mimpara may be used as part of a therapeutic regimen including phosphate binders and/or Vitamin D sterols, as appropriate (see section 5. 1).
Reduction of hypercalcaemia in patients with:
-parathyroid carcinoma.
- primary HPT for whom parathyroidectomy would be indicated on the basis of serum calcium
levels (as defined by relevant treatment guidelines), but in whom parathyroidectomy is not clinically appropriate or is contraindicated.
Treatment of parathyroid carcinoma, Treatment of primary hyperparathyroidism , Treatment of secondary hyperparathyroidism in patients with end-stage renal disease
Calcimimetic Agents
Small organic molecules that act as allosteric activators of the calcium sensing receptor (CaSR) in the PARATHYROID GLANDS and other tissues. They lower the threshold for CaSR activation by extracellular calcium ions and diminish PARATHYROID HORMONE (PTH) release from parathyroid cells. (See all compounds classified as Calcimimetic Agents.)
Calcium-Regulating Hormones and Agents
Hormones and molecules with calcium-regulating hormone-like actions that modulate OSTEOLYSIS and other extra-skeletal activities to maintain calcium homeostasis. (See all compounds classified as Calcium-Regulating Hormones and Agents.)
H05BX01
H05BX01
H05BX01
H05BX01