


1. Biquinate
2. Bisulfate, Quinine
3. Hydrochloride, Quinine
4. Legatrim
5. Myoquin
6. Quinamm
7. Quinbisan
8. Quinbisul
9. Quindan
10. Quinimax
11. Quinine
12. Quinine Bisulfate
13. Quinine Lafran
14. Quinine Sulfate
15. Quinine Sulphate
16. Quinine-odan
17. Quinoctal
18. Quinson
19. Quinsul
20. Strema
21. Sulfate, Quinine
22. Sulphate, Quinine
23. Surquina
1. Quinine Hcl
2. 130-89-2
3. Quinine Muriate
4. Chinimetten
5. Quinine Monohydrochloride
6. Fema No. 2976
7. Ccris 2002
8. Einecs 205-001-1
9. 7549-43-1
10. 7cs0wno31m
11. Anhydrous Quinine Hydrochloride
12. Quinine Hydrochloride Anhydrous
13. Ai3-62121
14. Quinine, Hydrochloride (2:1)
15. Quinine Hydrochloride Dihydrate
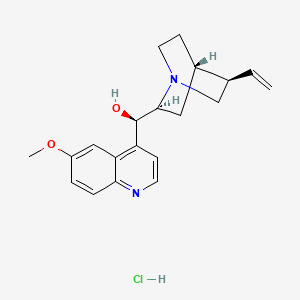
16. (r)-[(2s,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol;hydrochloride
17. Chininum Muriaticum
18. Quinine Chloride
19. Quinine Hydrochloride Hydrate
20. 6119-47-7
21. Cinchonan-9-ol, 6'-methoxy-, Hydrochloride (1:1), (8alpha,9r)-
22. (r)-(6-methoxyquinolin-4-yl)((1s,2s,4s,5r)-5-vinylquinuclidin-2-yl)methanol Hydrochloride
23. 30860-23-2
24. Chinine Hydrochloride
25. Quinine, Hydrochloride
26. Fema No. 2976, Dihydrate-
27. Einecs 231-437-7
28. Unii-7cs0wno31m
29. Quinine, Monohydrochloride
30. Quininehcl
31. Quinini Hydrochloridum
32. (8alpha,9r)-6'-methoxycinchonan-9-ol Monohydrochloride
33. 6'-methoxycinchonan-9-ol Monohydrochloride, (8alpha,9r)-
34. Cinchonan-9-ol, 6'-methoxy-, Monohydrochloride, (8alpha,9r)-
35. Quinine Hydrochloride (1:1)
36. Schembl184367
37. Chembl588046
38. Dtxsid7044213
39. Tcmdc-123484
40. Tcmdc-125479
41. Cinchonan-9-ol, 6'-methoxy-, Hydrochloride, (8alpha,9r)-
42. Cinchonan-9-ol, 6'-methoxy-, Hydrochloride, (8.alpha.,9r)-
43. Cinchonan-9-ol, 6'-methoxy-, Monohydrochloride, (8-alpha,9r)-
44. Quinine Hydrochloride [fhfi]
45. Mfcd00078498
46. Akos016002029
47. Cinchonan-9-ol, 6'-methoxy-, Monohydrochloride, Dihydrate, (8alpha,9r)-
48. Quinine Hydrochloride [who-dd]
49. 6'-methoxycinchonan-9-ol Hydrochloride
50. As-70722
51. Quinine Monohydrochloride Anhydrous
52. Quinine Hydrochloride Anhydrous [mi]
53. A51181
54. Q27268091
55. (8.alpha.,9r)-6'-methoxycinchonan-9-ol Hydrochloride
56. Cinchonan-9-ol, 6'-methoxy-, Hydrochloride (1:?), (8alpha,9r)-
57. (r)-(6-methoxyquinolin-4-yl)((2s,4s,5r)-5-vinylquinuclidin-2-yl)methanol Hydrochloride
58. (r)-[(2s,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](6-methoxyquinolin-4-yl)methanol Hydrochloride
| Molecular Weight | 360.9 g/mol |
|---|---|
| Molecular Formula | C20H25ClN2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 360.1604557 g/mol |
| Monoisotopic Mass | 360.1604557 g/mol |
| Topological Polar Surface Area | 45.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 457 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)

