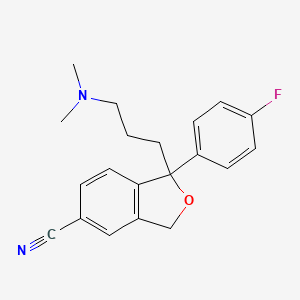

1. Celexa
2. Citalopram Hydrobromide
3. Cytalopram
4. Lu-10-171
5. Lu10171
6. Seropram
1. 59729-33-8
2. Nitalapram
3. Celexa
4. Cipram
5. Bonitrile
6. Citadur
7. 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
8. [3h]citalopram
9. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-2-benzofuran-5-carbonitrile
10. Lu-10-171
11. Chembl549
12. 1-(3-(dimethylamino)propyl)-1-(p-fluorophenyl)-5-phthalancarbonitrile
13. 0dhu5b8d6v
14. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3h-2-benzofuran-5-carbonitrile
15. Chebi:77397
16. [3h]-citalopram
17. Citalopramum [inn-latin]
18. Citalopramum
19. 5-isobenzofurancarbonitrile, 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydro-
20. 5-isobenzofurancarbonitrile, 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-
21. 5-isobenzofurancarbonitrile,1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-
22. Perrigo Citalopram
23. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile
24. Smr000465669
25. Citadur (tn)
26. Citalopram (usp/inn)
27. Einecs 261-891-1
28. Unii-0dhu5b8d6v
29. Citalopram [usp:inn:ban]
30. 1-[3-(dimethylamino)propyl]-1-(p-fluorophenyl)-5-phthalancarbonitrile
31. [3h]cytalopram
32. 1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile
33. [3h]escitalopram
34. Citalopram-[d4]
35. Citalopram-[d6]
36. [3h]lexapro
37. 1,3-dihydro-1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-5-isobenzofurancarbonitrile
38. Citalopram-d4 Oxalate
39. Escitalopram Impurity A
40. Citalopram [mi]
41. Citalopram [inn]
42. Prestwick3_000692
43. Schembl946
44. Citalopram [vandf]
45. Citalopram [mart.]
46. Citalopram [who-dd]
47. Lopac0_000258
48. Bspbio_000843
49. Mls000028578
50. Mls006011858
51. Bpbio1_000929
52. Gtpl4621
53. Gtpl7547
54. Dtxsid8022826
55. Bdbm25870
56. Hsdb 7042
57. Wseqxvzvjxjvfp-uhfffaoysa-
58. Citalopram [usp Impurity]
59. Hms2090o09
60. Hms2093a14
61. Hms3259b10
62. Act02666
63. Bbl029066
64. Lu-10171b
65. Stl058639
66. Akos005711003
67. Ac-8894
68. Ccg-204353
69. Db00215
70. Nc00711
71. Sdccgsbi-0050246.p003
72. 1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
73. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carb Onitrile
74. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3h-isobenzofuran-5-carbonitrile
75. 5-isobenzofurancarbonitrile, 1,3-dihydro-1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-
76. Mrf-0000302
77. Ncgc00015267-04
78. Ncgc00015267-05
79. Ncgc00015267-07
80. Ncgc00015267-09
81. Ncgc00015267-11
82. Ncgc00015267-12
83. Ncgc00015267-20
84. Ncgc00025160-02
85. As-76503
86. Sbi-0050246.p002
87. Hy-121203
88. Ab00513896
89. Cs-0081223
90. Ft-0657967
91. Ft-0660873
92. C07572
93. D07704
94. Ab00513896-09
95. Ab00513896-11
96. Ab00513896_12
97. Ab00513896_13
98. 729c338
99. A832440
100. L001223
101. Q409672
102. Sr-01000003129-8
103. Brd-a47598013-004-02-0
104. Brd-a47598013-004-06-1
105. 1-(3'-dimethylaminopropyl)-1-(4'-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
106. 1-(3-(dimethylamino)propyl)-1-(4'-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
107. 1-(3-dimethylamino-propyl)-1-(4-fluoro-phenyl)-1,3-dihydro-isobenzofuran-5-carbonitrile
108. 1-[3-(dimethylamino) Propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofuran Carbonitrile
109. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzo[b]furancarbonitrile
110. 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro[3,4]benzofuran-5-carbonitrile
111. 5-cyano-1-(4-fluorophenyl)-1-(3-dimethylaminopropyl)-1,3-dihydro-isobenzofuran
| Molecular Weight | 324.4 g/mol |
|---|---|
| Molecular Formula | C20H21FN2O |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 324.16379146 g/mol |
| Monoisotopic Mass | 324.16379146 g/mol |
| Topological Polar Surface Area | 36.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Celexa |
| PubMed Health | Citalopram (By mouth) |
| Drug Classes | Antidepressant |
| Active Ingredient | Citalopram hydrobromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Forest Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Celexa |
| PubMed Health | Citalopram (By mouth) |
| Drug Classes | Antidepressant |
| Active Ingredient | Citalopram hydrobromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Forest Labs |
Serotonin Uptake Inhibitors; Antidepressive Agents, Second-Generation
National Library of Medicine's Medical Subject Headings. Citalopram. Online file (MeSH, 2018). Available from, as of February 2, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Citalopram is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 2, 2018: https://clinicaltrials.gov/
Citalopram tablets are indicated for the treatment of depression. /Included in US product label/
NIH; DailyMed. Current Medication Information Citalopram Tablet, Film Coated (Updated: June 14, 2017). Available from, as of February 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6daeb45c-451d-b135-bf8f-2d6dff4b6b01
Citalopram has been used in the treatment of obsessive-compulsive disorder. /NOT included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2448
For more Therapeutic Uses (Complete) data for Citalopram (16 total), please visit the HSDB record page.
/BOXED WARNING/ SUICIDALITY AND ANTIDEPRESSANT DRUGS. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of citalopram or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Citalopram is not approved for use in pediatric patients.
NIH; DailyMed. Current Medication Information Citalopram Tablet, Film Coated (Updated: June 14, 2017). Available from, as of February 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6daeb45c-451d-b135-bf8f-2d6dff4b6b01
It is recommended that citalopram should not be used in patients with congenital long QT syndrome, bradycardia, hypokalemia or hypomagnesemia, recent acute myocardial infarction, or uncompensated heart failure. Citalopram should also not be used in patients who are taking other drugs that prolong the QTc interval. Such drugs include Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone).
NIH; DailyMed. Current Medication Information Citalopram Tablet, Film Coated (Updated: June 14, 2017). Available from, as of February 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6daeb45c-451d-b135-bf8f-2d6dff4b6b01
The citalopram dose should be limited in certain populations. The maximum dose should be limited ... in patients who are CYP2C19 poor metabolizers or those patients who may be taking concomitant cimetidine or another CYP2C19 inhibitor, since higher citalopram exposures would be expected. The maximum dose should also be limited ... in patients with hepatic impairment and in patients who are greater than 60 years of age because of expected higher exposures.
NIH; DailyMed. Current Medication Information Citalopram Tablet, Film Coated (Updated: June 14, 2017). Available from, as of February 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6daeb45c-451d-b135-bf8f-2d6dff4b6b01
The development of a potentially life-threatening serotonin syndrome has been reported with selective norepinephrine-reuptake inhibitors (SNRIs) and serotonin-reuptake inhibitors (SSRIs), including citalopram tablets, alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
NIH; DailyMed. Current Medication Information Citalopram Tablet, Film Coated (Updated: June 14, 2017). Available from, as of February 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6daeb45c-451d-b135-bf8f-2d6dff4b6b01
For more Drug Warnings (Complete) data for Citalopram (49 total), please visit the HSDB record page.
For the treatment of depression, as indicated by the FDA label. Off-label indications include but are not limited to: treatment of sexual dysfunction, post-stroke behavioural changes, ethanol abuse, obsessive-compulsive disorder (OCD) in children, and diabetic neuropathy,,,,,,,.
FDA Label
Citalopram belongs to a class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs). It has been found to relieve or manage symptoms of depression, anxiety, eating disorders and obsessive-compulsive disorder among other mood disorders. The antidepressant, anti-anxiety, and other actions of citalopram are linked to its inhibition of CNS central uptake of serotonin. Serotonergic abnormalities have been reported in patients with mood disorders. Behavioral and neuropsychological of effects of serotonin include the regulation of mood, perception, reward, anger, aggression, appetite, memory, sexuality, and attention, as examples. The onset of action for depression is approximately 1 to 4 weeks. The complete response may take 8-12 weeks after initiation of citalopram. In vitro studies demonstrate that citalopram is a strong and selective inhibitor of neuronal serotonin reuptake and has weak effects on norepinephrine and dopamine central reuptake. The chronic administration of citalopram has been shown to downregulate central norepinephrine receptors, similar to other drugs effective in the treatment of major depressive disorder. Citalopram does not inhibit monoamine oxidase.
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
N06AB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AB - Selective serotonin reuptake inhibitors
N06AB04 - Citalopram
Absorption
Rapidly and well absorbed from the GI tract. Peak plasma concentrations occur within 4 hours of a single orally administered dose. Bioavailability is 80% following oral administration. Food does not affect absorption.
Route of Elimination
12-23% of an oral dose of citalopram is found unchanged in the urine, while 10% of the dose is found in the faeces.
Volume of Distribution
12 L/kg Citalopram is highly lipophilic and likely widely distributed throughout the body, including the blood-brain-barrier. However, its metabolite, _demethylcitalopram_ does not penetrate the blood-brain-barrier well.
Clearance
The systemic clearance of citalopram is 330 mL/min, with approximately 20% renal clearance.
Like other selective serotonin-reuptake inhibitors, citalopram is a highly lipophilic compound that appears to be rapidly and well absorbed from the GI tract following oral administration. Following a single 40-mg oral dose of citalopram as a tablet, the manufacturer states that peak plasma concentrations averaging approximately 44 ng/mL occur at about 4 hours.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2460
The absolute bioavailability of citalopram is approximately 80% relative to an IV dose. The oral tablets and solution of citalopram reportedly are bioequivalent. Food does not substantially affect the absorption of citalopram.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2460
Distribution of citalopram and its metabolites into human body tissues and fluids has not been fully characterized. However, limited pharmacokinetic data suggest that the drug, which is highly lipophilic, is widely distributed in body tissues.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2460
/MILK/ /The purpose of the study was/ to characterize milk/plasma (M/P) ratio and infant dose, for citalopram and demethylcitalopram, in breast-feeding women taking citalopram for the treatment of depression, and to determine the plasma concentration and effects of these drugs in their infants. Seven women (mean age 30.6 years) taking citalopram (median dose 0.36 mg/kg/day) and their infants (mean age 4.1 months) were studied. Citalopram and demethylcitalopram in plasma and milk were measured by high-performance liquid chromatography over a 24 hr dose interval. Infant exposure was estimated (two separate methods) as the product of milk production rate and drug concentration in milk, normalized to body weight and expressed as a percentage of the weight-adjusted maternal dose. Mean M/PAUC values of 1.8 (range 1.2-3) and 1.8 (range 1.0-2.5) were calculated for citalopram and demethylcitalopram, respectively. The mean maximum concentrations of citalopram and demethylcitalopram in milk were 154 (95% CI, 102-207) ug/L and 50 (23-77) ug/L. Depending on the method of calculation, mean infant exposure was 3.2 or 3.7% for citalopram and 1.2 or 1.4% for demethylcitalopram. Citalopram (2.0, 2.3 and 2.3 ug/L) was detected in three of the seven infants. Demethylcitalopram (2.2 and 2.2 ug/L was detected in plasma from two of the same infants. ... The mean combined dose of citalopram and demethylcitalopram (4.4-5.1% as citalopram equivalents) transmitted to infants via breast milk is below the 10% notional level of concern. ...
PMID:10971311 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2014979 Rampono J et al; Br J Clin Pharmacol 50 (3): 263-8 (2000)
For more Absorption, Distribution and Excretion (Complete) data for Citalopram (14 total), please visit the HSDB record page.
Citalopram is metabolized mainly in the liver via N-demethylation to its main metabolite, _demethylcitalopram_ by CYP2C19 and CYP3A4. Other metabolites include _didemethylcitalopram_ via CYP2D6 metabolism, and _citalopram N-oxide_ via monoamine oxidase enzymes and aldehyde oxidase. It is a deaminated propionic acid derivative. After a single dose of citalopram, peak blood concentrations occur at approximately 4 hours. This drug in is found mainly unchanged in the plasma as citalopram. Cytochrome P450 (CYP) 3A4 and 2C19 isozymes appear to be heavily involved in producing _demethylcitalopram_. Demethylcitalopram appears to be further N-demethylated by CYP2D6 to didemethylcitalopram. Citalopram metabolites exert little pharmacologic activity in comparison to the parent drug and are not likely to contribute to the clinical effect of citalopram.
In a 2002 study, 11 women took citalopram (20-40 mg/day) during pregnancy; 10 throughtout gestation and 1 starting at 20 weeks' gestation. The mean ratio of two metabolites was significantly higher during pregnancy than at 2 months postdelivery, indicating induction of the CYP2D6 isoenzyme. The trough plasma concentration of citalopram, desmethylcitalopram, and didesmethylcitalopram in the normal new borns were 64%, 66%, and 68% of the maternal concentrations, respectively.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Tenth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2015, p. 282
The antidepressant citalopram (CT), a selective serotonin uptake inhibitor, was given in its labelled form, 14(C)-CT, as a single oral dose in 50 mL aqueous solution (0.1 mmol/30 uCi/1.1 MBq) to four healthy male volunteers. Concentrations of radioactivity in whole blood and plasma were similar. ... The HPLC profile of urinary components showed that besides the known metabolites of citalopram, three glucuronides were present. The relative amounts of CT and its metabolites in urine collected for 7 days were: CT (26 %), N-demethyl-CT (DCT, 19%), N,N-didemethyl-CT (DDCT,9%), the N-oxide (7%), the quaternary ammonium glucuronide of CT (CT-GLN, 14%), the N-glucuronide of DDCT (DDCT-GLN, 6%), and the glucuronide of the acid metabolite (CT-acid-GLN, 12%) formed by N,N-dimethyl deamination of CT. CT-GLN was isolated using preparative chromatography and identified by LC-MS-MS and NMR. DDCT-GLN and CT-acid-GLN were identified by LC-MS. This study shows that protracted renal excretion represents the major route of elimination, with a small fraction voided with feces. A considerable portion of the urinary excreted dose consists of N-glucuronides of CT and DDCT together with the O-acyl glucuronide of CT-acid.
PMID:10574684 Dalgaard L, Larsen C; Xenobiotica 29 (10): 1033-41 (1999)
This study was conducted to identify enzyme systems eventually catalyzing a local cerebral metab of citalopram. ... The metab of citalopram, of its enantiomers and demethylated metabolites was investigated in rat brain microsomes & in rat and human brain mitochondria. No cytochrome P-450 mediated transformation was observed in rat brain. ... In rat whole brain and in human frontal cortex, putamen, cerebellum & white matter of five brains monoamine oxidase activity was determined by the stereoselective measurement of the production of citalopram propionate. All substrates were metabolized by both forms of MAO, except in rat brain, where monoamine oxidase B activity could not be detected. Apparent Km and Vmax of S-citalopram biotransformation in human frontal cortex by monoamine oxidase B were found to be 266 uM & 6.0 pmol min/mg protein & by monoamine oxidase A 856 uM & 6.4 pmol min/mg protein, respectively. These Km values are in the same range as those for serotonin & dopamine metab by monoamine oxidases. ...
PMID:11840311 Kosel M, et al; Mol Psychiatry 7 (2): 181-8 (2002)
Citalopram ... is N-demethylated to N-desmethylcitalopram partially by CYP2C19 & partially by CYP3A4 & N-desmethylcitalopram is further N-demethylated by CYP2D6 to the likewise inactive metabolite di-desmethylcitalopram. The two metabolites are not active. ... In vitro citalopram does not inhibit CYP or does so only very moderately. A number of studies in healthy subjects and patients have confirmed, that this also holds true in vivo. Thus no change in pharmacokinetics or only very small changes were observed when citalopram was given with CYP1A2 substrates (clozapine & therophylline), CYP2C9 (warfarin), CYP2C19 (imipramine & mephenytoin), CYP2D6 (sparteine, imipramine & amitriptyline) and CYP3A4 (carbamazepine & triazolam). ...
PMID:11532381 Brosen K, Naranjo CA; Eur Neuropsychopharmacol 11 (4): 275-83 (2001)
For more Metabolism/Metabolites (Complete) data for Citalopram (7 total), please visit the HSDB record page.
Citalopram has known human metabolites that include Citalopram N-oxide and N-Desmethylcitalopram.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
About 35 hours.
The antidepressant citalopram (CT), a selective serotonin uptake inhibitor, was given in its labelled form, 14(C)-CT, as a single oral dose in 50 mL aqueous solution (0.1 mmol/30 uCi/1.1 MBq) to four healthy male volunteers. Concentrations of radioactivity in whole blood and plasma were similar. The respective pharmacokinetic parameters were: ... half life = 90.2+/-22.5 and 79.5 +/- 14.9 hr respectively. ...
PMID:10574684 Dalgaard L, Larsen C; Xenobiotica 29 (10): 1033-41 (1999)
The elimination half-life of citalopram averages approximately 35 hours in adults with normal renal and hepatic function.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2460
The mechanism of action of citalopram results from its inhibition of CNS neuronal reuptake of serotonin (5-HT). The molecular target for citalopram is the serotonin transporter (solute carrier family 6 member 4, _SLC6A4_), inhibiting its serotonin reuptake in the synaptic cleft. Citalopram binds with significantly less affinity to histamine, acetylcholine, and norepinephrine receptors than tricyclic antidepressant drugs. This drug has no or neglible affinity for _5-HT1A_, _5-HT2A_, _dopamine D_1 and _D2_, _1-_, _2_-, and_ adrenergic_, _histamine H1_, _gamma-aminobutyric acid_ (GABA), _muscarinic_, _cholinergic_, and _benzodiazepine_ receptors. Antagonism of _muscarinic_, _histaminergic_, and _adrenergic receptors_ is thought to be associated with several anticholinergic, sedative, and cardiovascular effects of other psychotropic drugs.
/In/ whole-cell patch clamp recording of heterologous HERG-mediated currents in transfected mammalian cells ... citalopram blocks HERG with an IC(50) of 3.97 uM. This is slightly less potent than fluoxetine in /the same/ system (IC(50) of 1.50 uM). In isolated guinea pig ventricular cardiomyocytes, citalopram inhibited L-type calcium current (I(Ca,L)). The voltage dependence of I(Ca,L) inactivation in the presence of 100 uM citalopram was shifted significantly leftward. As a result, the I(Ca,L) 'window' in citalopram was found to be smaller & leftward-shifted compared to control. /These/ effects ... may help to explain citalopram's good cardiac safety profile, given its propensity to block HERG at excessive dosages. /Salt not specified/
PMID:11852052 Witchel HJ, et al; FEBS Lett 512 (1-3): 59-66 (2002)
The study was aimed to investigate the effects of the minimal effective doses of acute citalopram (5 mg/kg), (+/-)-8-hydroxydipropylaminotetralin HBr (8-OH-DPAT; 0.1 mg/kg), & their combined treatment on the rat open field & forced swimming behaviour & post-mortem monoamine content. The animals were prospectively divided into the vehicle- and para-chlorophenylalanine (p-CPA)-pretreated (350 mg/kg) groups. Acute citalopram (5 mg/kg), 8-OH-DPAT (0.1 mg/kg), or their combined treatment had no major effect on the rat open field & forced swimming behaviour. The post-mortem catecholamine content in four brain regions studied was unchanged in all treatment groups. The combined 8-OH-DPAT (0.1 mg/kg) & citalopram (5 mg/kg) treatment partially reversed the p-CPA-induced decr of serotonin (5-HT) and 5-hydroxy-indolacetic acid (5-HIAA) content. The present experiments demonstrate that the 5-HT1A receptors mediate some of the selective serotonin reuptake inhibitor-induced biochemical phenomena.
PMID:11817498 Pruus K, et al; Prog Neuropsychopharmacol Biol Psychiatry 26 (2): 227-32 (2002)