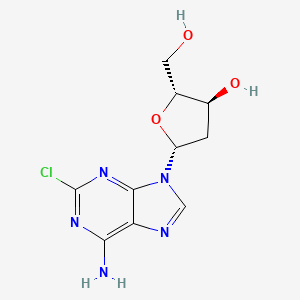

1. 2'-deoxy-2-chloroadenosine
2. 2-chloro-2'-deoxyadenosine
3. 2-chlorodeoxyadenosine
4. Leustatin
1. 2-chloro-2'-deoxyadenosine
2. 4291-63-8
3. Leustatin
4. 2-chlorodeoxyadenosine
5. 2-cda
6. Chlorodeoxyadenosine
7. Litak
8. Adenosine, 2-chloro-2'-deoxy-
9. Cldado
10. 2-chloro-2'-deoxy-beta-adenosine
11. Mavenclad
12. Cladaribine
13. 2cda
14. Rwj 26251
15. 2-chloro-deoxyadenosine
16. 2clado
17. Rwj-26251
18. Mls000028377
19. Cladarabine
20. Nsc-105014
21. (2r,3s,5r)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
22. Leustat
23. Smr000058553
24. (2r,3s,5r)-5-(6-amino-2-chloro-9h-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
25. (2r,3s,5r)-5-(6-amino-2-chloro-9h-purin-9-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol
26. 2-chloro-6-amino-9-(2-deoxy-beta-d-erythro-pentofuranosyl)purine
27. Chebi:567361
28. 47m74x9yt5
29. Movectro
30. Mylinax
31. Dsstox_cid_2828
32. Dsstox_rid_76747
33. Dsstox_gsid_22828
34. Cladribina
35. Cladribinum
36. Leustatin (tn)
37. Cl9
38. Cas-4291-63-8
39. 2 Chlorodeoxyadenosine
40. Sr-01000003063
41. Nsc 105014
42. Brn 0624220
43. Cladiribine
44. Unii-47m74x9yt5
45. Ccris 9374
46. Adenosine, 2-chloro-2'-deoxy
47. Hsdb 7564
48. 2-chloro-6-amino-9-(2-deoxy-beta-d-erythropentofuranosyl)purine
49. Cladribine [usan:usp:inn:ban]
50. Mavenclad (tn)
51. Ncgc00018167-03
52. Cladribine- Bio-x
53. Mfcd00153939
54. S1199
55. Cladribine [mi]
56. Rwj-26251-000
57. Cladribine [inn]
58. Cladribine [jan]
59. Opera_id_1191
60. Cladribine [hsdb]
61. Cladribine [usan]
62. Cladribine [vandf]
63. Cladribine [mart.]
64. Schembl3775
65. Chembl1619
66. Cladribine [usp-rs]
67. Cladribine [who-dd]
68. Cladribine (jan/usp/inn)
69. 2-chloro-2'-deoxy-adenosine
70. Cid_20279
71. Mls000028484
72. Mls000759397
73. Mls001077345
74. Mls001424194
75. Cladribine [ema Epar]
76. Gtpl4799
77. Cladribine [orange Book]
78. Dtxsid8022828
79. Bdbm38920
80. Cladribine [ep Monograph]
81. Cladribine [usp Impurity]
82. Cladribine For Peak Identification
83. Cladribine [usp Monograph]
84. Hms2052k13
85. Hms2232c23
86. Hms3715f17
87. 5542-92-7
88. Act02615
89. Amy22140
90. Bcp02868
91. Zinc3798064
92. Tox21_110834
93. Tox21_300596
94. Nsc-05014
95. Nsc-105014-f
96. Akos015854898
97. Akos015892544
98. Ac-7591
99. Bcp9000538
100. Ccg-101116
101. Cs-2057
102. Db00242
103. Nc00366
104. Ncgc00022567-05
105. Ncgc00022567-06
106. Ncgc00022567-07
107. Ncgc00022567-08
108. Ncgc00164384-01
109. Ncgc00254518-01
110. 2-chloro-2'-deoxyadenosine, Antileukemic
111. As-12366
112. Bc164318
113. Bp-25407
114. Hy-13599
115. Sw197746-4
116. D01370
117. Ab00382963-17
118. Ab00382963_19
119. 291c638
120. A826062
121. Q414030
122. 2-chloro-2 Inverted Exclamation Marka-deoxyadenosine
123. Sr-01000003063-7
124. Sr-01000003063-10
125. Cladribine, European Pharmacopoeia (ep) Reference Standard
126. 6-amino-2-chloro-9-(2-deoxy-beta-erythropentofuranosyl)purine
127. Cladribine, United States Pharmacopeia (usp) Reference Standard
128. 6-amino-2-chloro-9-(2-deoxy-beta-d-erythro-pentofuranosyl)purine
129. (2r,3s,5r)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxalan-3-ol
130. (2r,3s,5r)-5-(6-amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol
131. Cladribine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
132. 2-chloro-2'-deoxyadenosine;(2r,3s,5r)-5-(6-amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)tetrahydrofuran-3-ol
133. 24757-90-2
| Molecular Weight | 285.69 g/mol |
|---|---|
| Molecular Formula | C10H12ClN5O3 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 285.0628670 g/mol |
| Monoisotopic Mass | 285.0628670 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 338 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cladribine |
| PubMed Health | Cladribine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Cladribine Injection (also commonly known as 2-chloro-2-deoxy--D-adenosine) is a synthetic antineoplastic agent for continuous intravenous infusion. It is a clear, colorless, sterile, preservative-free, isotonic solution. Cladribine injectio... |
| Active Ingredient | Cladribine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Onco Therapies; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Cladribine |
| PubMed Health | Cladribine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Cladribine Injection (also commonly known as 2-chloro-2-deoxy--D-adenosine) is a synthetic antineoplastic agent for continuous intravenous infusion. It is a clear, colorless, sterile, preservative-free, isotonic solution. Cladribine injectio... |
| Active Ingredient | Cladribine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Onco Therapies; Eurohlth Intl |
Antineoplastic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Cladribine is indicated for active treatment of hairy cell leukemia as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Cladribine is accepted for treatment of B-cell chronic lymphocytic leukemia (CLL) in both previously untreated patients and patients refractory to previous treatment, based upon reports of objective tumor responses, most of which were partial, in noncomparative studies. /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Cladribine is accepted for treatment of low-grade non-Hodgkin's lymphomas in patients refractory to previous treatment, based upon reports of objective tumor responses, most of which were partial, in two noncomparative studies. /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Cladribine is accepted for treatment of Waldenstrom macroglobulinemia, based upon reports of objective tumor responses in one noncomparative study. /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
The most common adverse effects of cladribine in patients with hairy cell leukemia during the first month after initiation of therapy in clinical trials were severe neutropenia, fever (often culture negative), and documented infection. Myelosuppression, which may be severe, usually is reversible, and appears to be dose dependent, should be anticipated with use of the drug. At recommended doses, cladribine appears to be rarely associated with many adverse effects that frequently occur with antineoplastic therapy (e.g., nausea, vomiting, hair loss, abnormal renal or hepatic function). The most frequent adverse nonhematologic effects of the drug that occur during the first 2 weeks after initiation of therapy are fatigue, nausea, rash, headache, and reactions at the injection site. Most adverse nonhematologic effects are mild to moderate in severity.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979
Severe bone marrow suppression resulting in neutropenia, anemia, and thrombocytopenia occurs frequently in patients with hairy cell leukemia receiving cladribine, especially at high doses or in patients with preexisting pancytopenia. Most patients with hairy cell leukemia receiving cladribine in clinical trials had hematologic impairment as a manifestation of the disease. Following cladribine treatment, further hematologic impairment occurred before recovery of peripheral blood counts began. Prolonged pancytopenia including aplastic anemia and hemolytic anemia (reported in patients with lymphoid malignancies within the first few weeks following cladribine therapy) has been reported in postmarketing surveillance of patients usually receiving multiple courses of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979
Myelosuppression occurred frequently during the first month after initiation of cladribine therapy in patients with hairy cell leukemia in clinical trials; 44% of patients received red blood cell transfusions and 14% received platelet transfusions. During the first 2 weeks after treatment was initiated, mean platelet count, absolute neutrophil count (ANC), and hemoglobin concentration declined and subsequently increased with normalization of mean counts by day 12, week 5, and week 8, respectively. Platelet recovery may be delayed in patients with severe baseline thrombocytopenia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979
Multiple cycles of cladribine therapy may be associated with cumulative myelotoxicity and prolonged thrombocytopenia. Thrombocytopenia was the limiting toxicity in 20-30% of patients with chronic lymphocytic leukemia or lymphomas after repeated courses of cladribine therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979
For more Drug Warnings (Complete) data for CLADRIBINE (32 total), please visit the HSDB record page.
For the treatment of active hairy cell leukemia (leukemic reticuloendotheliosis) as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms. Also used as an alternative agent for the treatment of chronic lymphocytic leukemia (CLL), low-grade non-Hodgkin's lymphoma, and cutaneous T-cell lymphoma.
Treatment of adult patients with highly active relapsing multiple sclerosis (MS) as defined by clinical or imaging features.
Litak is indicated for the treatment of hairy-cell leukaemia.
Multiple Sclerosis
Cladribine is a synthetic purine nucleoside that acts as an antineoplastic agent with immunosuppressive effects. Cladribine differs structurally from deoxyadenosine only by the presence of a chlorine atom at position 2 of the purine ring, which results in resistance to enzymatic degradation by adenosine deaminase. Due to this resistance, cladribine exhibits a more prolonged cytotoxic effect than deoxyadenosine against resting and proliferating lymphocytes. Cladribine is one of a group of chemotherapy drugs known as the anti-metabolites. Anti-metabolites stop cells from making and repairing DNA, which are processes that are necessary for cancer cells to grow and multiply.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
L04AA40
L01BB04
L01BB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BB - Purine analogues
L01BB04 - Cladribine
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA40 - Cladribine
Absorption
Oral bioavailability is 34 to 48%.
Volume of Distribution
4.5 2.8 L/kg [patients with hematologic malignancies]
9 L/kg
Clearance
978 +/- 422 mL/h/kg
Cladribine is bound approximately 20% to plasma proteins.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2357
It is not known whether cladribine is distributed into breast milk.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
It is not known whether cladribine is removed from circulation by dialysis or hemofiltration.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979
Cladribine penetrates into cerebrospinal fluid. One report indicates that concentrations are approximately 25% of those in plasma.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2357
For more Absorption, Distribution and Excretion (Complete) data for CLADRIBINE (9 total), please visit the HSDB record page.
Metabolized in all cells with deoxycytidine kinase activity to 2-chloro-2'-deoxyadenosine-5'-triphosphate
Metabolized in all cells with deoxycytidine kinase activity to 2-chloro-2'-deoxyadenosine-5'-triphosphate.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
5.4 hours
Cladribine plasma concentration after intravenous administration declines multi-exponentially with an average half-life of 6.7 +/-2.5 hours.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2357
... The terminal half-life varies from 5.7 to 19.7 hours ...
PMID:9068927 Liliemark J; Clin Pharmacokinet 32 (2): 120-31 (1997)
... The terminal phase half-life in 22 patients ranged from 14.3-25.8 hr, with a mean (SD) of 19.7 (3.4) hr. ...
PMID:7906999 Kearns C, Blakley R et al; Cancer Res 54 (5): 1235-9 (1994)
Cladribine is structurally related to fludarabine and pentostatin but has a different mechanism of action. Although the exact mechanism of action has not been fully determined, evidence shows that cladribine is phosphorylated by deoxycytidine kinase to the nucleotidecladribine triphosphate (CdATP; 2-chloro-2-deoxyadenosine 5-triphosphate), which accumulates and is incorporated into DNA in cells such as lymphocytes that contain high levels of deoxycytidine kinase and low levels of deoxynucleotidase, resulting in DNA strand breakage and inhibition of DNA synthesis and repair. High levels of CdATP also appear to inhibit ribonucleotide reductase, which leads to an imbalance in triphosphorylated deoxynucleotide (dNTP) pools and subsequent DNA strand breaks, inhibition of DNA synthesis and repair, nicotinamide adenine dinucleotide (NAD) and ATP depletion, and cell death. Unlike other antimetabolite drugs, cladribine has cytotoxic effects on resting as well as proliferating lymphocytes. However, it does cause cells to accumulate at the G1/S phase junction, suggesting that cytotoxicity is associated with events critical to cell entry into S phase. It also binds purine nucleoside phosphorylase (PNP), however no relationship between this binding and a mechanism of action has been established.
Cladribine is an antimetabolite. The exact mechanism of action in hairy cell leukemia is unknown. Cladribine is resistant to the action of adenosine deaminase (ADA), which deaminates deoxyadenosine to deoxyinosine. The phosphorylated metabolites of cladribine accumulate in cells with a high ratio of deoxycytidine kinase activity to 5' nucleotidase activity (lymphocytes, monocytes ) and are converted to the active triphosphate deoxynucleotide. Intracellular accumulation of toxic deoxynucleotides selectively kills these cells, which become unable to properly repair single-strand DNA breaks, leading to disruption of cell metabolism. In addition, there is some evidence that deoxynucleotides are incorporated into the DNA of dividing cells and impair DNA synthesis. Cladribine also induces apoptosis (a form of programmed cell death in sensitive cells). Cladribine's action is cell cycle-phase nonspecific; cladribine equally affects dividing and resting lymphocytes.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Cladribine has immunosuppressant activity ; restoration of lymphocyte subsets after treatment takes at least 6 to 12 months, although clinical immunocompetence is usually restored after about a month. Significant reductions in T and B lymphocytes occur during treatment (both CD4 and CD8 are affected) and CD4 counts recover more slowly after treatment.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
/Investigators/ have studied the role of caspases and mitochondria in apoptosis induced by 2-chloro-2'-deoxyadenosine (cladribine) in several human leukemic cell lines. Cladribine treatment induced mitochondrial transmembrane potential (DeltaPsi(m)) loss, phosphatidylserine exposure, caspase activation and development of typical apoptotic morphology in JM1 (pre-B), Jurkat (T) and U937 (promonocytic) cells. Western-blot analysis of cell extracts revealed the activation of at least caspases 3, 6, 8 and 9. Co-treatment with Z-VAD-fmk (benzyloxy-carbonyl-Val-Ala-Asp-fluoromethylketone), a general caspase inhibitor, significantly prevented cladribine-induced death in JM1 and Jurkat cells for the first approximately 40 h, but not for longer times. Z-VAD-fmk also partly prevented some morphological and biochemical features of apoptosis in U937 cells, but not cell death. Co-incubation with selective caspase inhibitors Ac-DEVD-CHO (N-acetyl-Asp-Glu-Val-Asp-aldehyde), Ac-LEHD-CHO (N-acetyl-Leu-Glu-His-Asp-aldehyde) or Z-IETD-fmk (benzyloxycarbonyl-Ile-Glu-Thr-Asp-fluoromethylketone), inhibition of protein synthesis with cycloheximide or cell-cycle arrest with aphidicolin did not prevent cell death. Overexpression of Bcl-2, but not CrmA, efficiently prevented death in Jurkat cells. In all cell lines, death was always preceded by Delta Psi(m) loss and accompanied by the translocation of the protein apoptosis-inducing factor (AIF) from mitochondria to the nucleus. These results suggest that caspases are differentially involved in induction and execution of apoptosis depending on the leukemic cell lineage. In any case, Delta Psi(m) loss marked the point of no return in apoptosis and may be caused by two different pathways, one caspase-dependent and the other caspase-independent. Execution of apoptosis was always performed after Delta Psi(m) loss by a caspase-9-triggered caspase cascade and the action of AIF.
PMID:11672427 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1222174 Marzo I, Perez-Galan P et al; Biochem J 359 (Pt 3): 537-46 (2001)
Cladribine (chlorodeoxyadenosine, 2-CdA), a synthetic purine nucleoside, is an antineoplastic agent. ... The precise mechanism(s) of antileukemic action of cladribine has not been fully elucidated. Cladribine is phosphorylated by deoxycytidine kinase to the nucleotide cladribine triphosphate (CdATP; 2-chloro-2'-deoxyadenosine 5'-triphosphate), which accumulates and is incorporated into DNA in cells such as lymphocytes that have high levels of deoxycytidine kinase and low levels of deoxynucleotidase. High intracellular concentrations of cladribine triphosphate appear to inhibit ribonucleotide reductase, causing an imbalance in triphosphorylated deoxynucleotide (dNTP) pools and subsequent DNA strand breaks, inhibition of DNA synthesis and repair, nicotinamide adenine dinucleotide (NAD) and ATP depletion, and cell death. Incorporation of accumulated cladribine triphosphate into DNA also may contribute to DNA strand breakage and inhibition of DNA synthesis and repair. Unlike other commonly used antineoplastic drugs that affect purine and pyrimidine metabolism, cladribine has cytotoxic effects on resting as well as proliferating lymphocytes and monocytes.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 979