


1. Afazol Grin
2. Ak Con
3. Ak-con
4. Albalon
5. All Clear
6. Clear Eyes
7. Colirio Alfa
8. Hydrochloride, Naphazoline
9. Idril
10. Miraclar
11. Monohydrochloride, Naphazoline
12. Nafazair
13. Naphazoline Hydrochloride
14. Naphazoline Monohydrochloride
15. Naphazoline Nitrate
16. Naphcon
17. Naphcon Forte
18. Nitrate, Naphazoline
19. Optazine
20. Pensa, Vasoconstrictor
21. Privin
22. Privine
23. Proculin
24. Siozwo
25. Tele Stulln
26. Tele-stulln
27. Vasoclear
28. Vasocon
29. Vasocon Regular
30. Vasoconstrictor Pensa
31. Vasonit
1. 835-31-4
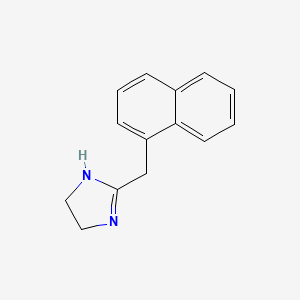
2. 2-(naphthalen-1-ylmethyl)-4,5-dihydro-1h-imidazole
3. Naphthizine
4. Antan
5. Nafazolin
6. Ciba 2020/r
7. 2-(1-naphthylmethyl)-2-imidazoline
8. 1h-imidazole, 4,5-dihydro-2-(1-naphthalenylmethyl)-
9. Nafazair
10. 2-(naphthyl-(1')-methyl)imidazolin
11. Naphcon
12. Naphazoline (inn)
13. 2-imidazoline, 2-(1-naphthylmethyl)-
14. H231gf11bv
15. Clearine
16. 2-(1-naphthylmethyl)-4,5-dihydro-1h-imidazole
17. 2-[(naphthalen-1-yl)methyl]-4,5-dihydro-1h-imidazole
18. Nafazolina [dcit]
19. Naphazolinum
20. Nafazolina
21. Nafazoline
22. Nafazoline [spanish]
23. Naphazolinum [latin]
24. Naphazoline [inn]
25. Naphazoline [inn:ban]
26. Naphazolinum [inn-latin]
27. Rhinazine
28. Alpha-naphthylmethyl Imidazoline
29. Chembl1706
30. 2-(alpha-naphthylmethyl)-imidazoline
31. Nafazolin (tn)
32. 4,5-dihydro-2-(1-naphthylmethyl)imidazole
33. Enamine_000333
34. 10061-11-7
35. 2-(naphthyl-(1')-methyl)imidazolin [german]
36. Einecs 212-641-5
37. Brn 0151864
38. Unii-h231gf11bv
39. Imidin
40. Privine (salt/mix)
41. Sanorin (salt/mix)
42. Spectrum_000975
43. Naphazoline [mi]
44. Prestwick0_000046
45. Prestwick1_000046
46. Prestwick2_000046
47. Prestwick3_000046
48. Spectrum2_001054
49. Spectrum3_000513
50. Spectrum4_000068
51. Spectrum5_001424
52. Chembl761
53. Naphazoline [vandf]
54. Naphazoline [mart.]
55. Schembl34532
56. Bspbio_000171
57. Bspbio_002065
58. Kbiogr_000595
59. Kbioss_001455
60. Naphazoline [who-dd]
61. 5-23-08-00293 (beilstein Handbook Reference)
62. Cid_11079
63. Divk1c_000456
64. Spbio_001008
65. Spbio_002092
66. Bpbio1_000189
67. Gtpl5509
68. Dtxsid3048449
69. Chebi:93363
70. Kbio1_000456
71. Kbio2_001455
72. Kbio2_004023
73. Kbio2_006591
74. Kbio3_001565
75. Ninds_000456
76. .alpha.-naphthylmethyl Imidazoline
77. Hms1394p03
78. Hms2090d15
79. Zinc119717
80. Bbl012612
81. Bdbm50001922
82. Mfcd00066737
83. Stk300042
84. Akos000295230
85. Ccg-250339
86. Db06711
87. 2-(.alpha.-naphthylmethyl)-imidazoline
88. 2-(1-naphthalenylmethyl)-2-imidazoline
89. Idi1_000456
90. Ncgc00016506-01
91. Ncgc00016506-02
92. Ncgc00016506-03
93. Ncgc00016506-04
94. Ncgc00016506-05
95. Vs-03410
96. Sbi-0051454.p003
97. Db-050155
98. Hy-111326
99. Cs-0034952
100. Ft-0725973
101. D08253
102. Ab00053505-11
103. Ab00053505-12
104. Ab00053505_13
105. Ab00053505_14
106. 550n992
107. A840596
108. L000878
109. Q415433
110. 2-(1-naphthalenylmethyl)-4,5-dihydro-1h-imidazole
111. 2-(1-naphthylmethyl)-4,5-dihydro-1h-imidazole #
112. Brd-k77641333-003-05-7
113. Brd-k77641333-003-15-6
114. Brd-k77641333-003-25-5
115. Z56762888
116. 2-(1-naphthylmethyl)-4,5-dihydro-1h-imidazole;naphazoline
117. 2-naphthalen-1-ylmethyl-4,5-dihydro-1h-imidazole; Hydrochloride
| Molecular Weight | 210.27 g/mol |
|---|---|
| Molecular Formula | C14H14N2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 210.115698455 g/mol |
| Monoisotopic Mass | 210.115698455 g/mol |
| Topological Polar Surface Area | 24.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 272 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | Naphcon-a |
| Active Ingredient | <a class="pubchem-internal-link CID-11079" href="/compound/Naphazoline%20hydrochloride">Naphazoline hydrochloride</a>; <a class="pubchem-internal-link CID-52821 |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.025%; 0.3% |
| Market Status | Over the Counter |
| Company | Alcon |
Naphazoline is indicated for use as OTC eyedrops for ocular vasoconstriction or as a nasal preparation for nasal congestion.
Naphazoline is a sympathomimetic alpha adrenergic agonist that acts to vasoconstrict nasal or ocular arterioles, resulting in reduced congestion at the site of administration.
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993) (See all compounds classified as Nasal Decongestants.)
Adrenergic alpha-Agonists
Drugs that selectively bind to and activate alpha adrenergic receptors. (See all compounds classified as Adrenergic alpha-Agonists.)
R01AA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AA - Sympathomimetics, plain
R01AA08 - Naphazoline
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AB - Sympathomimetics, combinations excl. corticosteroids
R01AB02 - Naphazoline
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GA - Sympathomimetics used as decongestants
S01GA01 - Naphazoline
Absorption
Absorption data for naphazoline are scarce but imidazoline compounds in general are weakly basic and lipophilic, with high bioavailability from the gastrointestinal tract.
Route of Elimination
Imidazoline compounds undergo some hepatic metabolism but a large fraction of the dose may be excreted unchanged in the urine. Urinary excretion is higher with more acidic urine.
Volume of Distribution
Distribution data for naphazoline are scarce but imidazoline compounds are distributed throughout the body, and can cross the blood-brain barrier.
Clearance
Clearance data for naphazoline is unavailable.
Metabolism data for naphazoline are scarce. Imidazoline compounds undergo some hepatic metabolism but a large fraction of the dose may be excreted unchanged in the urine.
Half life has not been determined but effects last for 4 to 8 hours. Other imidazoline compounds have half lives varying from 2 to 12 hours.
Naphazoline is a vasoconstrictor that functions by stimulating alpha adrenergic receptors in arterioles leading to decreased congestion at the site of administration. Naphazoline causes the release of norepinephrine in sympathetic nerves. Norepinephrine binds to alpha adrenergic receptors and causes vasoconstriction. Naphazoline is also a mild beta adrenergic receptor agonist, which can cause rebound vasodilation after the alpha adrenergic stimulation has ended. Naphazoline's release of norepinephrine also triggers a negative feedback loop which decreases production of norepinephrine, which can lead to rhinitis medicamentosa after long term use when naphazoline is stopped.