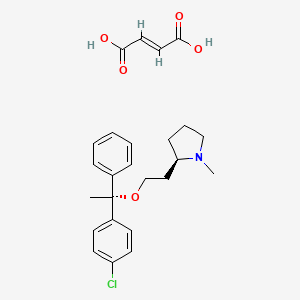

1. 2-(2-(1-(4-chlorophenyl)-1-phenylethoxy)ethyl)-1-methylpyrrolidine
2. Clemastine
3. Hs 592
4. Hs-592
5. Hs592
6. Meclastine
7. Mecloprodin
8. Tavegyl
9. Tavist
1. 14976-57-9
2. Tavist
3. Agasten
4. Clemastine Hydrogen Fumarate
5. Meclastine Fumarate
6. Tavegil
7. Clemastine (fumarate)
8. (r)-2-(2-((r)-1-(4-chlorophenyl)-1-phenylethoxy)ethyl)-1-methylpyrrolidine Fumarate
9. Chebi:3739
10. Mecloprodine
11. Aloginan
12. Xolamin
13. 19259egq3d
14. (+)-(2r)-2-(2-(((r)-p-chloro-alpha-methyl-alpha-phenylbenzyl)oxy)ethyl)-1-methylpyrrolidine Fumarate (1:1)
15. 14976-57-9 (fumarate)
16. Alphamin
17. Anhistan
18. Clemanil
19. Fuluminol
20. Inbestan
21. Kinotomin
22. Lacretin
23. Lecasol
24. Maikohis
25. Marsthine
26. Masletine
27. Piloral
28. Reconin
29. Tavegyl
30. Trabest
31. Mallermin-f
32. Telgin-g
33. Dsstox_cid_27765
34. Dsstox_rid_82542
35. Dsstox_gsid_47785
36. Tavist-1
37. Pyrrolidine, 2-(2-(1-(4-chlorophenyl)-1-phenylethoxy)ethyl)-1-methyl-, (r-(r*,r*))-, (e)-2-butenedioate (1:1)
38. Meclastine Hydrogen Fumarate
39. (2e)-but-2-enedioic Acid; (2r)-2-{2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine
40. (e)-but-2-enedioic Acid;(2r)-2-[2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine
41. Hsdb 6507
42. Topcare Dayhist Allergy
43. Ncgc00016710-01
44. Contac 12 Hour Allergy
45. Einecs 239-055-2
46. Cas-14976-57-9
47. Clemastine For System Suitability
48. Unii-19259egq3d
49. (r)-2-[2-[(r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine Fumarate
50. Prestwick_680
51. Meclastine (fumarate)
52. Clemastinefumarate,(s)
53. Clemastine Fumarate [usan:usp:ban:jan]
54. Hs-592 (fumarate)
55. (+)-2-(2-((p-chloro-alpha-methyl-alpha-phenylbenzyl)oxy)ethyl)-1-methyl Pyrrolidine Fumarate
56. Schembl33403
57. Schembl41468
58. Spectrum1500191
59. Chembl1200795
60. Dtxsid6047785
61. Hms500o07
62. Hy-b0298a
63. Clemastine Fumarate [jan]
64. Clemastine Fumarate [hsdb]
65. Clemastine Fumarate [usan]
66. Hms1568n14
67. Hms1920o13
68. Hms2091e20
69. Hms2095n14
70. Hms3267l12
71. Hms3412b19
72. Hms3676b19
73. Hms3712n14
74. Hms3884k12
75. Pharmakon1600-01500191
76. Clemastine Fumarate [vandf]
77. Bcp27945
78. Clemastine Fumarate [mart.]
79. Tox21_110574
80. Ccg-40028
81. Clemastine Fumarate [usp-rs]
82. Clemastine Fumarate [who-dd]
83. Mfcd00137486
84. Nsc756685
85. S1847
86. Akos015896237
87. Akos037643271
88. Tox21_110574_1
89. Ac-1338
90. Clemastine Fumarate [orange Book]
91. Clemastine Hydrogen Fumarate [mi]
92. Ncgc00016710-05
93. Ncgc00180903-01
94. Ncgc00180903-02
95. (r-(r*,r*))-2-(2-(1-(p-chlorophenyl)-1-phenylethoxy)ethyl)-1-methylpyrrolidinium Hydrogen Fumarate
96. As-12093
97. Bc164321
98. Clemastine Fumarate [ep Monograph]
99. Pyrrolidine, 2-(2-((p-chloro-alpha-methyl-alpha-phenylbenzyl)oxy)ethyl)-1-methyl-, (+)-, Fumarate (1:1)
100. Clemastine Fumarate [usp Monograph]
101. Clemastine Fumarate Salt, >=98% (hplc)
102. Sw196835-3
103. Tavist-d Component Clemastine Fumarate
104. H10994
105. Clemastine Fumarate Component Of Tavist-d
106. 976c579
107. Sr-05000001590
108. Sr-05000001590-1
109. Q27106180
110. Clemastine Fumarate, European Pharmacopoeia (ep) Reference Standard
111. Clemastine Fumarate, United States Pharmacopeia (usp) Reference Standard
112. (2r)-2-[2-[(1r)-1-(4-chlorophenylethoxy]ethyl]-1-methyl-2-pyrrolidine Fumarate
113. Clemastine For System Suitability, European Pharmacopoeia (ep) Reference Standard
114. (+)-(2r)-2-(2-(((r)-p-chloro-.alpha.-methyl-.alpha.-phenylbenzyl)oxy)ethyl)-1-methylpyrrolidine Fumarate (1:1)
115. (2r)-2-[2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidin-1-ium;(e)-4-hydroxy-4-oxobut-2-enoate
116. (2r)-2-{2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine (2e)-but-2-enedioate
117. (2r)-2-{2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidinium (2e)-3-carboxyprop-2-enoate
118. Pyrrolidine,2-[2-[(1r)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methyl-, (2r)-,(2e)-2-butenedioate (1:1)
| Molecular Weight | 460.0 g/mol |
|---|---|
| Molecular Formula | C25H30ClNO5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 459.1812508 g/mol |
| Monoisotopic Mass | 459.1812508 g/mol |
| Topological Polar Surface Area | 87.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Clemastine fumarate |
| Active Ingredient | Clemastine fumarate |
| Dosage Form | Tablet; Syrup |
| Route | Oral |
| Strength | 1.34mg; 2.68mg; eq 0.5mg base/5ml |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Teva; Perrigo; Sandoz |
| 2 of 4 | |
|---|---|
| Drug Name | Tavist-1 |
| Drug Label | Clemastine belongs to the benzhydryl ether group of antihistaminic compounds. The chemical name is (+)-(2R)-2-[2-[[(R)-p-Chloro--methyl--phenylbenzyl]-oxy]ethyl]-1-methylpyrrolidine fumarate (1:1).C21H26C1NO.C4H4O4 M.W. 459.97Each tablet for oral... |
| Active Ingredient | Clemastine fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.34mg |
| Market Status | Over the Counter |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Clemastine fumarate |
| Active Ingredient | Clemastine fumarate |
| Dosage Form | Tablet; Syrup |
| Route | Oral |
| Strength | 1.34mg; 2.68mg; eq 0.5mg base/5ml |
| Market Status | Over the Counter; Prescription |
| Company | Wockhardt; Teva; Perrigo; Sandoz |
| 4 of 4 | |
|---|---|
| Drug Name | Tavist-1 |
| Drug Label | Clemastine belongs to the benzhydryl ether group of antihistaminic compounds. The chemical name is (+)-(2R)-2-[2-[[(R)-p-Chloro--methyl--phenylbenzyl]-oxy]ethyl]-1-methylpyrrolidine fumarate (1:1).C21H26C1NO.C4H4O4 M.W. 459.97Each tablet for oral... |
| Active Ingredient | Clemastine fumarate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.34mg |
| Market Status | Over the Counter |
| Company | Novartis |
For the symptomatic treatment of allergic rhinitis in adults and children 12 years of age and older ...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 15
For the symptomatic treatment of mild allergic urticaria and angioedema in adults and children 12 years of age and older ...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 15
This multicenter, double blind, randomized parallel group study compared 3 wk treatment with either loratadine (Clarityn) 10 mg once daily, or clemastine (Tavegyl) 1 mg twice daily, and placebo in outpatients with active perennial allergic rhinitis. 155 patients were evaluated for efficacy and safety. Grading of four nasal and three non-nasal symptoms, rhinoscopy signs, and therapeutic response was performed on treatment days 6, 13, and 20. Patients recorded daily symptoms and possible adverse experiences in a diary, also indicating when symptoms of active rhinitis were relieved. Loratadine and clemastine were statistically significantly superior to placebo throughout the study (p < 0.05), based on assessment of patients' nasal and eye symptoms, patients' diary scores, rhinoscopy signs of symptoms, and onset of relief. The loratadine group showed a statistically significantly (p < 0.05) faster onset of relief of symptoms compared with the group treated with clemastine. Concerning nasal stuffiness, loratadine was significantly (p < 0.05) superior to clemastine after 1 week's treatment. Reports of adverse reactions showed that significantly (p < 0.05) more patients complained of sedation in the clemastine than in the loratadine group. Regarding other adverse experiences and laboratory tests, the three treatment groups were statistically comparable (p < 0.05). The study showed that compared with placebo both loratadine and clemastine were effective in relieving nasal and eye symptoms in patients with perennial allergic rhinitis. Loratadine was safe and well tolerated and was significantly less sedative than clemastine; loratadine may therefore possess an advantage in clinical use in the treatment of perennial allergic rhinitis.
PMID:2143361 Frolund L et al; Allergy 45 (4): 254-61 (1990)
The effects of cromolyn sodium (sodium cromoglycate), clemastine and ketotifen administered to the nasal mucosa of seasonal and perennial allergic rhinitis patients 30 min before provocation with histamine and allergen were compared in a randomized, double blind, placebo controlled study. Clemastine and cromolyn sodium, but not ketotifen, significantly inhibited the nasal response to increasing concentrations of histamine. None of the drugs administered in the concentrations used significantly inhibited the nasal response to allergen.
PMID:3117084 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1386248 Corrado OJ et al; Br J Clin Pharmacol 24 (Sep): 283-92 (1987)
Drugs contraindicated during lactation include ... clemastine.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 113
There are no adequate and controlled studies to date using clemastine fumarate alone or in fixed combination with phenylpropanolamine in pregnant women, and the drug should be used during pregnancy only when clearly needed.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 15
Because of the potential for serious adverse reactions to clemastine in nursing infants, a decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 15
Drugs that have been associated with Significant Effects on some Nursing Infants and should be given to Nursing Mothers with Caution: Clemastine: Drowsiness, irritability, refusal to feed, high-pitched cry, neck stiffness (1 case). /from Table 5/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 139 (1994)
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
In 12 healthy subjects given clemastine fumarate by mouth peak plasma concentrations occurred after 3 to 5 hr. After intravenous injection in 3 subjects a rapid decline in plasma concentration during the first 30 minutes occurred followed by a slow rise to peak concentration 2 to 3 hours after administraton. In 5 subjects given clemastine fumarate the ability to inhibit histamine flares correlated well with plasma concentation.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1307
Clemastine fumarate is rapidly and almost completely absorbed from the GI tract. Peak plasma concentrations of the drug are attained within 2-5 hours after a single oral dose. Following oral administration of clemastine fumarate, the antihistaminic effect of the drug (as measured by suppression of the wheal response induced by intradermal injection of histamine) is maximal within 5-7 hours and persists for 10-12 and, in some individuals, up to 24 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 14
Distribution of clemastine into human body fluids and tissues has not been fully characterized, but the drug has been shown to distribute into milk. Following oral administration of 2.68 mg of clemastine fumarate daily for 3 days in one nursing woman, a milk clemastine concentration of about 5-10 ug/ml occurred 20 hours after the last dose of the drug and was about 25-50% of the stimultaneous maternal plasma drug concentration; clemastine was undetectable in milk 6 days after discontuance of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 14
Clemastine and its metabolites are eliminated principally in urine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 14
The exact metabolic fate of clemastine is not clearly established, but the drug appears to be extensively metabolized. Clemastine and its metabolites are eliminated principally in urine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 14
Time to peak concentration: 2-4 hr
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.I p.403 (1992)
Inhibitory effects of various histamine receptor-blocking agents including antiallergic agents on metabolic activations of neutrophils were examined by measuring leukotrienes (C4, D4 and E4) formation, arachidonic acid release and superoxide generation. The stimulation of neutrophils by calcium ionophore causes the production of leukotrienes concomitantly with the release of arachidonic acid from the cells and these were effectively diminished with a variety of antihistaminic agents. Almost all the histamine H1 receptor-blocking drugs studied here showed as inhibitors of the metabolic activations of neutrophils, although the degree was dependent on the drug concentrations. The order of potency of the inhibitory effects on the arachidonic acid release were: homochlorcyclizine, clemastine, and azelastine (IC50 < 20 microM) greater than oxatomide (IC50 < 60 microM) and diphenylpyraline greater than triprolidine, meclizine, diphenhydramine (IC50 > 100 microM). The superoxide generation from neutrophils activated by phorbol 12-myristate 13-acetate was also effectively inhibited by these agents, but generally lower concentrations were required to obtain the same degrees of effects. These results indicated that some of the histamine H1 receptor-blocking drugs, such as clemastine and homochlorcyclizine, may act as inhibitors of formation of leukotrienes at the locus of inflammation.
PMID:1678430 Taniguchi K et al; J Pharmacobiodyn 14 (2): 87-93 (1991)