


1. 7-chloro-7-deoxylincomycin Palmitate
2. 7-chlorolincomycin Palmitate
3. Cleocin Palmitate
4. Clindamycin Palmitate
1. Clindamycin Palmitate Hcl
2. 25507-04-4
3. Cleocin Pediatric
4. Clindamycin 2-palmitate Hydrochloride
5. Vn9a8jm7m7
6. U-25,179 E
7. 25507-04-4 (palmitate Hcl)
8. Nsc-759141
9. U-25179e
10. U-25179-e
11. Clindamycin Palmitate (hydrochloride)
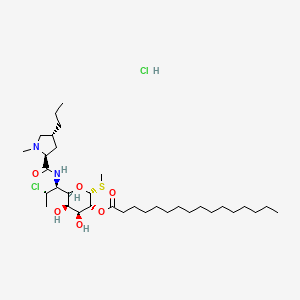
12. (2r,3r,4s,5r,6r)-6-((1s,2s)-2-chloro-1-((2s,4r)-1-methyl-4-propylpyrrolidine-2-carboxamido)propyl)-4,5-dihydroxy-2-(methylthio)tetrahydro-2h-pyran-3-yl Palmitate
13. (2r,3r,4s,5r,6r)-6-((1s,2s)-2-chloro-1-((2s,4r)-1-methyl-4-propylpyrrolidine-2-carboxamido)propyl)-4,5-dihydroxy-2-(methylthio)tetrahydro-2h-pyran-3-yl Palmitate Hydrochloride
14. [(2r,3r,4s,5r,6r)-6-[(1s,2s)-2-chloro-1-[[(2s,4r)-1-methyl-4-propylpyrrolidine-2-carbonyl]amino]propyl]-4,5-dihydroxy-2-methylsulfanyloxan-3-yl] Hexadecanoate;hydrochloride
15. Unii-vn9a8jm7m7
16. Ncgc00185773-02
17. U 25179e
18. Cleocin Pediatric (tn)
19. Dsstox_cid_28660
20. Dsstox_rid_82930
21. Dsstox_gsid_48734
22. Schembl41416
23. Clindamycin Palmitate Hydrochloride [usan:usp:jan]
24. Chembl1200632
25. Dtxsid3048734
26. Niosh/gf2450000
27. Chebi:34647
28. Hy-b1454
29. Tox21_113175
30. Mfcd09752824
31. Akos030242182
32. Ccg-270385
33. Nsc 759141
34. As-58470
35. Clindamycin-2-palmitate, Monohydrochloride
36. Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-l-2-pyrrolidinecarboxamido)-1-thio-l-threo-alpha-d-galacto-octopyranoside 2-palmitate Monohydrochloride
37. Cas-25507-04-4
38. Cs-0013162
39. Gf24500000
40. Clindamycin Palmitate Hydrochloride (jan/usp)
41. D01990
42. F20748
43. Clindamycin Palmitate Hydrochloride [jan]
44. Clindamycin Palmitate Hydrochloride [mart.]
45. Clindamycin Palmitate Hydrochloride [usan]
46. Clindamycin Palmitate Hydrochloride [vandf]
47. Clindamycin Palmitate Hydrochloride [usp-rs]
48. Clindamycin Palmitate Hydrochloride [who-dd]
49. J-016026
50. Q27291919
51. Clindamycin Palmitate Hydrochloride [orange Book]
52. Clindamycin Palmitate Hydrochloride [usp Monograph]
53. L-threo-.alpha.-d-galacto-octopyranoside, Methyl 7-chloro-6,7,8-trideoxy-6-(((1-methyl-4-propyl-2-pyrrolidinyl)carbonyl)amino)-1-thio-2-hexadecanoate, Monohydrochloride, (2s-trans)-
54. L-threo-alpha-d-galacto-octopyranoside, Methyl 7-chloro-6,7,8-trideoxy-6-(((1-methyl-4-propyl-2-pyrrolidinyl)carbonyl)amino)-1-thio-2-hexadecanoate, Monohydrochloride, (2s-trans)-
55. Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-l-2-pyrrolidinecarboxamido)-1-thio-l-threo-.alpha.-d-galacto-octopyranoside 2-palmitate Monohydrochloride
| Molecular Weight | 699.9 g/mol |
|---|---|
| Molecular Formula | C34H64Cl2N2O6S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 23 |
| Exact Mass | 698.3862143 g/mol |
| Monoisotopic Mass | 698.3862143 g/mol |
| Topological Polar Surface Area | 134 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 810 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Clindamycin palmitate hydrochloride |
| Drug Label | Clindamycin palmitate hydrochloride is a water soluble hydrochloride salt of the ester of clindamycin and palmitic acid. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compoun... |
| Active Ingredient | Clindamycin palmitate hydrochloride |
| Dosage Form | For solution |
| Route | Oral |
| Strength | eq 75mg base/5ml |
| Market Status | Prescription |
| Company | Lyne; Amneal Pharms; Paddock Labs; Aurobindo Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Clindamycin palmitate hydrochloride |
| Drug Label | Clindamycin palmitate hydrochloride is a water soluble hydrochloride salt of the ester of clindamycin and palmitic acid. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compoun... |
| Active Ingredient | Clindamycin palmitate hydrochloride |
| Dosage Form | For solution |
| Route | Oral |
| Strength | eq 75mg base/5ml |
| Market Status | Prescription |
| Company | Lyne; Amneal Pharms; Paddock Labs; Aurobindo Pharma |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)