


1. Cl 68
2. Clocortolone Trimethylacetate
3. Cloderm
4. Purantix
1. 34097-16-0
2. Clocortolone 21-pivalate
3. Cloderm
4. Sh 863
5. Qbl8izh14x
6. Purantix
7. Clocortolone Pivalate (200 Mg)
8. Mls002154165
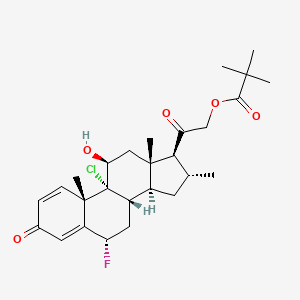
9. [2-[(6s,8s,9r,10s,11s,13s,14s,16r,17s)-9-chloro-6-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-7,8,11,12,14,15,16,17-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 2,2-dimethylpropanoate
10. Chebi:59583
11. Sh-863
12. 9-chloro-6alpha-fluoro-11beta,21-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 21-pivalate
13. Cilder
14. Cl 68
15. 9-chloro-6alpha-fluoro-11beta-hydroxy-16alpha-methyl-3,20-dioxopregna-1,4-dien-21-yl 2,2-dimethylpropanoate
16. Unii-qbl8izh14x
17. Clocortolone Pivalate [usan]
18. Clocortolone Pivalate [usan:usp]
19. Ncgc00016824-01
20. Pregna-1,4-diene-3,20-dione, 9-chloro-21-(2,2-dimethyl-1-oxopropoxy)-6-fluoro-11-hydroxy-16-methyl-, (6.alpha.,11.beta.,16.alpha.)-
21. Clocortolone-pivalate
22. Cloderm (tn)
23. Einecs 251-826-5
24. Cas-34097-16-0
25. Prestwick0_001119
26. Prestwick1_001119
27. Prestwick2_001119
28. Prestwick3_001119
29. Schembl5120
30. Dsstox_cid_25460
31. Dsstox_rid_80891
32. Clocortolone Pivalate (usp)
33. Dsstox_gsid_45460
34. Bspbio_001258
35. Spbio_003119
36. Bpbio1_001383
37. Chembl1200975
38. Dtxsid0045460
39. Hms1571o20
40. Hms2098o20
41. Hms2236m06
42. Hms3715o20
43. 9-chloro-6.alpha.-fluoro-11.beta.,21-dihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione 21-pivalate
44. Zinc4212603
45. Tox21_110632
46. Clocortolone Pivalate [vandf]
47. Clocortolone Pivalate [mart.]
48. Clocortolone Pivalate [usp-rs]
49. Clocortolone Pivalate [who-dd]
50. Ccg-221119
51. Clocortolone 21-pivalate [mi]
52. Ncgc00179239-01
53. Ncgc00179239-05
54. Smr001233464
55. Clocortolone Pivalate [orange Book]
56. Ab00514058
57. Clocortolone Pivalate [usp Monograph]
58. D02287
59. Sr-01000838872
60. Sr-01000838872-2
61. Brd-k38003476-001-03-4
62. Q39045318
63. (6alpha,11beta,16alpha)-9-chloro-21-(2,2-dimethyl-1-oxopropoxy)-6-fluoro-11-hydroxy-16-methypregna-1,4-diene-3,20-dione
64. 9-chloro-6
65. A-fluoro-11
66. A,21-dihydroxy-16
67. A-methylpregna-1,4-diene-3,20-dione 21-pivalate Pound Clocortolone Pivalate)
68. 9alpha-chloro-6alpha-fluoro-11beta,21-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 21-pivalate
69. Pregna-1,4-diene-3,20-dione, 9-chloro-21-(2,2-dimethyl-1-oxopropoxy)-6-fluoro-11-hydroxy-16-methyl-, (6alpha,11beta,16alpha)-
| Molecular Weight | 495.0 g/mol |
|---|---|
| Molecular Formula | C27H36ClFO5 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 494.2235301 g/mol |
| Monoisotopic Mass | 494.2235301 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 982 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cloderm |
| PubMed Health | Clocortolone Pivalate (On the skin) |
| Drug Classes | Corticosteroid, Weak |
| Drug Label | Cloderm Cream 0.1% contains the medium potency topical corticosteroid, clocortolone pivalate, in a specially formulated water-washable emollient cream base consisting of purified water, white petrolatum, mineral oil, stearyl alcohol, polyoxyl 40 stea... |
| Active Ingredient | Clocortolone pivalate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Promius Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Cloderm |
| PubMed Health | Clocortolone Pivalate (On the skin) |
| Drug Classes | Corticosteroid, Weak |
| Drug Label | Cloderm Cream 0.1% contains the medium potency topical corticosteroid, clocortolone pivalate, in a specially formulated water-washable emollient cream base consisting of purified water, white petrolatum, mineral oil, stearyl alcohol, polyoxyl 40 stea... |
| Active Ingredient | Clocortolone pivalate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Promius Pharma |
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)