

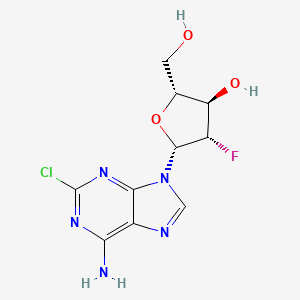

1. 2 Chloro 2' Arabino Fluoro 2' Deoxyadenosine
2. 2 Chloro 2' Fluoroarabino 2' Deoxyadenosine
3. 2-chloro-2'-arabino-fluoro-2'-deoxyadenosine
4. 2-chloro-2'-fluoroarabino-2'-deoxyadenosine
5. 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arbinofuranosyl)adenine
6. 2-chloro-9-(2-deoxy-2-fluoroarabinofuranosyl)adenine
7. 9h-purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-
8. Cl-f-ara-a
9. Clofarex
10. Clolar
11. Evoltra
1. 123318-82-1
2. Clolar
3. Evoltra
4. Clofarex
5. Cafda
6. Cl-f-ara-a
7. C1-f-ara-a
8. (2r,3r,4s,5r)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol
9. 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-9h-purin-6-amine
10. 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)adenine
11. Chebi:681569
12. (2r,3r,4s,5r)-5-(6-amino-2-chloro-9h-purin-9-yl)-4-fluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol
13. 762rdy0y2h
14. 9h-purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-
15. (2r,3r,4s,5r)-5-(6-amino-2-chloro-9h-purin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol
16. Nsc-759857
17. Clofarabine [usan]
18. 2-chloro-9-(2'-deoxy-2'-fluoro-beta-d-arabinofuranosyl)adenine
19. Dsstox_cid_26437
20. Dsstox_rid_81613
21. Dsstox_gsid_46437
22. Cfb
23. Clofarabina
24. Clofarabinum
25. Cl-f-araa
26. Mfcd00871077
27. 2-chloro-9-(2-deoxy-2-fluoro-b -d-arabinofuranosyl)-9h-purin-6-amine
28. (2r,3r,4s,5r)-5-(6-amino-2-chloro-purin-9-yl)-4-fluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol
29. Clolar (tn)
30. Cas-123318-82-1
31. 2-cl-2'-f-araa
32. Unii-762rdy0y2h
33. Clofarabine [usan:inn:ban]
34. Ncgc00164553-01
35. Clofarabine [mi]
36. Clofarabine [inn]
37. Clofarabine [jan]
38. 2-chloro-9-(2-deoxy-2-fluoro-b-d-arabinofuranosyl)-9h-purin-6-amine
39. 2-chloro-2'-arabino-fluoro-2'-deoxyadenosine
40. Clofarabine [vandf]
41. Clofarabine,clolar, Evoltra
42. Schembl9040
43. Chembl1750
44. Clofarabine [mart.]
45. Clofarabine [who-dd]
46. Mls003915615
47. Clofarabine (jan/usan/inn)
48. Clofarabine [ema Epar]
49. Gtpl6802
50. Dtxsid5046437
51. Clofarabine, >=98% (hplc)
52. Clofarabine [orange Book]
53. Hms2090a07
54. Hms3413j15
55. Hms3677j15
56. Act05522
57. Bcp23422
58. Hy-a0005
59. Zinc3798247
60. Tox21_112182
61. Ac-274
62. Bdbm50247921
63. S1218
64. Akos005063562
65. Akos015919355
66. Tox21_112182_1
67. Bcp9000540
68. Ccg-264865
69. Cs-0373
70. Db00631
71. Nsc 759857
72. Sar-393590
73. Ncgc00164553-02
74. Ncgc00164553-05
75. As-12958
76. Smr002530055
77. Bcp0726000280
78. Sw218080-2
79. D03546
80. Ab00430247-03
81. Ab00430247-04
82. Ab00430247_06
83. 318c821
84. Sr-01000930565
85. Q5134875
86. Sr-01000930565-3
87. Z1557400138
88. 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-adenine
89. 2-chloro-9-(2-deoxy-2-fluoro-?-d-arabinofuranosyl)-9h-purin-6-amine
90. 9h-purine-6-amine,2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-
91. 2-chloro-9-(2-deoxy-2-fluoro-.beta.-d-arabinofuranosyl)-9h-purin-6-amine
92. 9h-purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-.beta.-d-arabinofuranosyl)
93. (2r,3r,4s,5r)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol 2-chloro-2''-arabino-fluoro-2''-deoxyadenosine
| Molecular Weight | 303.68 g/mol |
|---|---|
| Molecular Formula | C10H11ClFN5O3 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 303.0534451 g/mol |
| Monoisotopic Mass | 303.0534451 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Clolar |
| PubMed Health | Clofarabine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Clolar (clofarabine) Injection contains clofarabine, a purine nucleoside metabolic inhibitor. Clolar (1 mg/mL) is supplied in a 20 mL, single-use vial. The 20 mL vial contains 20 mg clofarabine formulated in 20 mL unbuffered normal saline (comprised... |
| Active Ingredient | Clofarabine |
| Dosage Form | Injectable |
| Route | Iv (infusion) |
| Strength | 20mg/20ml (1mg/ml) |
| Market Status | Prescription |
| Company | Genzyme |
| 2 of 2 | |
|---|---|
| Drug Name | Clolar |
| PubMed Health | Clofarabine (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Clolar (clofarabine) Injection contains clofarabine, a purine nucleoside metabolic inhibitor. Clolar (1 mg/mL) is supplied in a 20 mL, single-use vial. The 20 mL vial contains 20 mg clofarabine formulated in 20 mL unbuffered normal saline (comprised... |
| Active Ingredient | Clofarabine |
| Dosage Form | Injectable |
| Route | Iv (infusion) |
| Strength | 20mg/20ml (1mg/ml) |
| Market Status | Prescription |
| Company | Genzyme |
For the treatment of pediatric patients 1 to 21 years old with relapsed or refractory acute lymphocytic (lymphoblastic) leukemia after at least two prior regimens. It is designated as an orphan drug by the FDA for this use.
FDA Label
Treatment of acute lymphoblastic leukaemia (ALL) in paediatric patients who have relapsed or are refractory after receiving at least two prior regimens and where there is no other treatment option anticipated to result in a durable response. Safety and efficacy have been assessed in studies of patients 21 years old at initial diagnosis.
Clofarabine is a purine nucleoside antimetabolite that differs from other puring nucleoside analogs by the presence of a chlorine in the purine ring and a flourine in the ribose moiety. Clofarabine seems to interfere with the growth of cancer cells, which are eventually destroyed. Since the growth of normal body cells may also be affected by clofarabine, other effects also occur. Clofarabine prevents cells from making DNA and RNA by interfering with the synthesis of nucleic acids, thus stopping the growth of cancer cells.
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
L01BB06
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BB - Purine analogues
L01BB06 - Clofarabine
Route of Elimination
Based on 24-hour urine collections in the pediatric studies, 49 - 60% of the dose is excreted in the urine unchanged.
Volume of Distribution
172 L/m2
Clearance
28.8 L/h/m2 [Pediatric patients (2 - 19 years old) with relapsed or refractory acute lymphoblastic leukemia (ALL) or acute myelogenous leukemia (AML) receiving 52 mg/m2 dose]
Clofarabine is sequentially metabolized intracellularly to the 5’-monophosphate metabolite by deoxycytidine kinase and mono- and di-phosphokinases to the active 5’-triphosphate metabolite. Clofarabine has high affinity for the activating phosphorylating enzyme, deoxycytidine kinase, equal to or greater than that of the natural substrate, deoxycytidine.
The terminal half-life is estimated to be 5.2 hours.
Clofarabine is metabolized intracellularly to the active 5'-monophosphate metabolite by deoxycytidine kinase and 5'-triphosphate metabolite by mono- and di-phospho-kinases. This metabolite inhibits DNA synthesis through an inhibitory action on ribonucleotide reductase, and by terminating DNA chain elongation and inhibiting repair through competitive inhibition of DNA polymerases. This leads to the depletion of the intracellular deoxynucleotide triphosphate pool and the self-potentiation of clofarabine triphosphate incorporation into DNA, thereby intensifying the effectiveness of DNA synthesis inhibition. The affinity of clofarabine triphosphate for these enzymes is similar to or greater than that of deoxyadenosine triphosphate. In preclinical models, clofarabine has demonstrated the ability to inhibit DNA repair by incorporation into the DNA chain during the repair process. Clofarabine 5'-triphosphate also disrupts the integrity of mitochondrial membrane, leading to the release of the pro-apoptotic mitochondrial proteins, cytochrome C and apoptosis-inducing factor, leading to programmed cell death.
