

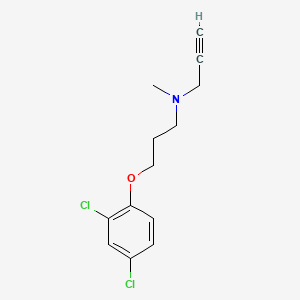

1. Chlorgyline
2. Clorgilin
3. Clorgyline
1. Clorgyline
2. 17780-72-2
3. Chlorgyline
4. Clorgilinum
5. Clorgilina
6. M And B 9302
7. Clorgiline [inn]
8. M & B 9302
9. N-methyl-n-propargyl-3-(2,4-dichlorophenoxy)propylamine
10. N-(3-(2,4-dichlorophenoxy)propyl)-n-methyl-2-propynylamine
11. M&b 9302
12. Clorgiline (inn)
13. Lyj16fzu9q
14. N-(3-(2,4-dichlorophenoxy)propyl)-n-methylprop-2-yn-1-amine
15. Chembl8706
16. Chebi:3763
17. N-[3-(2,4-dichlorophenoxy)propyl]-n-methylprop-2-yn-1-amine
18. 2-propyn-1-amine, N-(3-(2,4-dichlorophenoxy)propyl)-n-methyl-
19. 2-propynylamine, N-(3-(2,4-dichlorophenoxy)propyl)-n-methyl-
20. Clorgilinum [inn-latin]
21. Clorgilina [inn-spanish]
22. 2-propyn-1-amine, N-(3-(2,4-dichlorophenoxy)propyl)-n-methyl- (9ci)
23. Unii-lyj16fzu9q
24. Cas-17780-75-5
25. Brn 1976758
26. 2-propyn-1-amine, N-[3-(2,4-dichlorophenoxy)propyl]-n-methyl-, Hydrochloride
27. 3-(2,4-dichlorophenoxy)-n-methyl-n-prop-2-ynylpropan-1-amine
28. Clorgiline [inn:ban]
29. Prestwick0_000344
30. Prestwick1_000344
31. Prestwick2_000344
32. Prestwick3_000344
33. Lopac-m-3778
34. Lopac0_000746
35. Schembl61914
36. Bspbio_000407
37. Us8633208, Clorgyline
38. Spbio_002328
39. Bpbio1_000449
40. Gtpl6636
41. Dtxsid3048445
42. Bdbm15581
43. Zinc22200090
44. Ccg-204831
45. Db04017
46. Sdccgsbi-0050724.p003
47. Ncgc00015669-01
48. Ncgc00015669-02
49. Ncgc00015669-03
50. Ncgc00015669-04
51. Ncgc00015669-11
52. Ncgc00162235-01
53. M&b-9302
54. Sbi-0050724.p002
55. A20772
56. D03248
57. L001342
58. Q2889652
59. Brd-k73251053-003-03-2
60. Brd-k73251053-003-13-1
61. [3-(2,4-dichloro-phenoxy)-propyl]-methyl-prop-2-ynyl-amine
62. [3-(2,4-dichlorophenoxy)propyl](methyl)prop-2-yn-1-ylamine
63. N-[3-(2,4-dichlorophenoxy)propyl]-n-methyl-prop-2-yn-1-amine
64. 3-(2,4-dichlorophenoxy)-n-methyl-n-prop-2-ynyl-propan-1-amine
65. N-[(2,6-dichloro-4-methyl-3-pyridyl)carbonyl]-n-(3,4-dichlorophenyl)urea
66. Clg
| Molecular Weight | 272.17 g/mol |
|---|---|
| Molecular Formula | C13H15Cl2NO |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 271.0530695 g/mol |
| Monoisotopic Mass | 271.0530695 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 263 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Monoamine Oxidase Inhibitors
A chemically heterogeneous group of drugs that have in common the ability to block oxidative deamination of naturally occurring monoamines. (From Gilman, et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed, p414) (See all compounds classified as Monoamine Oxidase Inhibitors.)
