


1. Y 6047
2. Y-6047
3. Y6047
1. Rize
2. Trecalmo
3. Rizen
4. Tienor
5. Rise
6. 33671-46-4
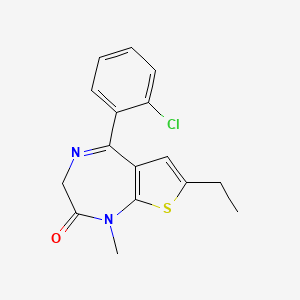
7. Clotiazepamum [inn-latin]
8. Y 6047
9. 5-(2-chlorophenyl)-7-ethyl-1-methyl-3h-thieno[2,3-e][1,4]diazepin-2-one
10. 5-(o-chlorophenyl)-7-ethyl-1,3-dihydro-1-methyl-2h-thieno(2,3-e)-1,4-diazepin-2-one
11. 5-(2-chlorophenyl)-7-ethyl-1-methyl-1,3-dihydro-2h-thieno(2,3-e)(1,4)diazepin-2-one
12. Zcn055599v
13. 2h-thieno(2,3e)(1,4)-diazepin-2-one, 5-(o-chlorophenyl)-7-ethyl-1,3-dihydro-1-methyl-
14. 5-(2-chlorophenyl)-7-ethyl-1-methyl-1,3-dihydro-2h-thieno[2,3-e][1,4]diazepin-2-one
15. Clotiazepamum
16. Distensan
17. Veratran
18. Clozan
19. Clotiazepam [inn:jan]
20. 2h-thieno(2,3-e)(1,4)diazepin-2-one, 1,3-dihydro-5-(o-chlorophenyl)-7-ethyl-1-methyl-
21. 2h-thieno[2,3-e]-1,4-diazepin-2-one, 5-(2-chlorophenyl)-7-ethyl-1,3-dihydro-1-methyl-
22. Rize (tn)
23. Einecs 251-627-3
24. Brn 0623332
25. Unii-zcn055599v
26. Dea No. 2752
27. Y-6047
28. Clotiazepam [mi]
29. Clotiazepam [inn]
30. Clotiazepam [jan]
31. Clotiazepam (jp17/inn)
32. Clotiazepam [mart.]
33. Schembl44592
34. Clotiazepam [who-dd]
35. Zinc1207
36. Chembl1697737
37. Dtxsid0022852
38. Chebi:31425
39. Hy-u00317
40. Akos005065735
41. Cs-7319
42. Db01559
43. D01328
44. Q2263999
45. 2h-thieno[2,3-e]-1,4-diazepine-2-one, 5-(2-chlorophenyl)-7-ethyl-1,3-dihydro-1-methyl-
46. 5-(2-chlorophenyl)-7-ethyl-1-methyl-1h,2h,3h-thieno[2,3-e][1,4]diazepin-2-one
| Molecular Weight | 318.8 g/mol |
|---|---|
| Molecular Formula | C16H15ClN2OS |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 318.0593620 g/mol |
| Monoisotopic Mass | 318.0593620 g/mol |
| Topological Polar Surface Area | 60.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of anxiety disorders.
Clotiazepam is a thienodiazepine possessing anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. It increases the stage 2 non-rapid eye movement sleep.
N05BA21
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA21 - Clotiazepam
Hepatic.
Clotiazepam has known human metabolites that include Hydroxy-clotiazepam and desmethyl-clotiazepam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4 hours
Clotiazepam acts at the benzodiazepine receptors (BZD). This agonizes the action of GABA, increasing the frequency of opening of the channel chlorinates and penetration of the ions chlorinates through the ionophore. Increase in membrane polarization decreases the probability of discharge of neurons.