


1. 31104, Gt
2. Cholestagel
3. Colesevelam Hcl
4. Colesevelam Hydrochloride
5. Gt 31104
6. Gt-31104
7. Gt31 104
8. Gt31-104
9. Gt31104
10. Hcl, Colesevelam
11. Hydrochloride, Colesevelam
12. Welchol
1. Welchol
2. Colesevelam Hcl
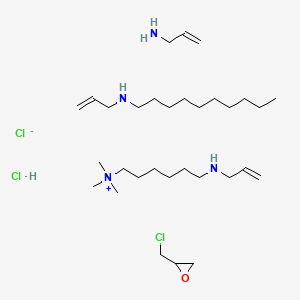
3. 2-(chloromethyl)oxirane;prop-2-en-1-amine;n-prop-2-enyldecan-1-amine;trimethyl-[6-(prop-2-enylamino)hexyl]azanium;chloride;hydrochloride
4. Gt 31-104hb
5. Unii-p4sg24wi5q
6. Gt-31104
7. Gt 31-104
8. Colesevelam Hydrochloride [usan]
9. P4sg24wi5q
10. Gt31-104hb
11. Cholestagel, Colesevelam Hydrochloride
12. Akos037653488
13. Hs-0084
14. Nsc 760126
15. 1-hexaminium, N,n,n-trimethyl-6-(2-propenylamino)-, Chloride, Polymer With (chloromethyl)oxirane, 2-propen-4-amine And N-2-propenyl-1-decanamine, Hydrochloride
16. 2-propen-1-amine Polymer With (chloromethyl)oxirane, N,n,n-trimethyl-6-(2-propenylamino)-1-hexanaminium Chloride, And N-2-propenyl-1-decanamine, Hydrochloride
17. Allylamine Polymer With 1-chloro-2,3-epoxypropane, (6-(allylamino)hexyl)trimethylammonium Chloride And N-allyldecylamine, Hydrochloride
18. Ft-0697863
19. Ab01563397_01
20. Q899036
| Molecular Weight | 618.2 g/mol |
|---|---|
| Molecular Formula | C31H67Cl3N4O |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 22 |
| Exact Mass | 616.438046 g/mol |
| Monoisotopic Mass | 616.438046 g/mol |
| Topological Polar Surface Area | 62.6 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 301 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 6 |
| 1 of 2 | |
|---|---|
| Drug Name | Welchol |
| PubMed Health | Colesevelam (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | WELCHOL (colesevelam hydrochloride) is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent intended for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule. Colesevelam hydrochloride is poly(a... |
| Active Ingredient | Colesevelam hydrochloride |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 625mg; 1.875gm/packet; 3.75gm/packet |
| Market Status | Prescription |
| Company | Daiichi Sankyo |
| 2 of 2 | |
|---|---|
| Drug Name | Welchol |
| PubMed Health | Colesevelam (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | WELCHOL (colesevelam hydrochloride) is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent intended for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule. Colesevelam hydrochloride is poly(a... |
| Active Ingredient | Colesevelam hydrochloride |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | 625mg; 1.875gm/packet; 3.75gm/packet |
| Market Status | Prescription |
| Company | Daiichi Sankyo |
For use, alone or in combination with an HMG-CoA reductase inhibitor, as adjunctive therapy to diet and exercise for the reduction of elevated LDL cholesterol in patients with primary hypercholesterolemia (Fredrickson Type IIa).
FDA Label
Cholestagel co-administered with a 3-hydroxy-3-methyl-glutaryl-coenzyme-A (HMG-CoA)-reductase inhibitor (statin) is indicated as adjunctive therapy to diet to provide an additive reduction in low-density-lipoprotein-cholesterol (LDL-C) levels in adult patients with primary hypercholesterolaemia who are not adequately controlled with a statin alone.
Cholestagel as monotherapy is indicated as adjunctive therapy to diet for reduction of elevated total cholesterol and LDL-C in adult patients with primary hypercholesterolaemia, in whom a statin is considered inappropriate or is not well tolerated.
Cholestagel can also be used in combination with ezetimibe, with or without a statin, in adult patients with primary hypercholesterolaemia, including patients with familial hypercholesterolaemia (see section 5. 1).
Colesevelam is a high capacity bile-acid binding molecule. Colesevelam binds to bile acids in the intestine which reduces the amount of bile acids that are returned to the liver via enterohepatic circulation. Clinical studies have demonstrated that elevated levels of total cholesterol (total-C), LDL-C, and apolipoprotein B (Apo B, a protein associated with LDL-C) are associated with an increased risk of atherosclerosis in humans. Similarly, decreased levels of high-density lipoprotein cholesterol (HDL-C) are associated with the development of atherosclerosis. Epidemiological investigations have established that cardiovascular morbidity and mortality vary directly with the levels of total-C and LDL-C, and inversely with the level of HDL-C. The combination of colesevelam and an HMG-CoA reductase inhibitor is effective in further lowering serum total-C and LDL-C levels beyond that achieved by either agent alone.
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
C10AC04
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AC - Bile acid sequestrants
C10AC04 - Colesevelam
Absorption
Not hydrolyzed by digestive enzymes and is not absorbed.
Route of Elimination
Excretion: In 16 healthy volunteers, an average of 0.05% of administered radioactivity from a single 14C-labeled colesevelam hydrochloride dose was excreted in the urine.
Not applicable (not hydrolyzed by digestive enzymes and not absorbed).
Colesevelam binds bile acids in the intestine and prevents their reabsorption. Colesevelam is not absorbed itself. The depletion of bile acid causes the upregulation of cholesterol 7-alpha-hydroxylase and conversion of cholesterol to bile acid. this increases the production and activity of hydroxymethyl-glutaryl-coenzyme A (HMG-CoA) reductase in the liver as well as an increase in the number of low density lipoprotein (LDL) receptors. This process clears LDL cholesterol from the blood. Serum triglyceride levels may increase or remain unchanged. The end result is increased clearance of LDL-cholesterol from the blood with decreased serum LDL-cholesterol.