

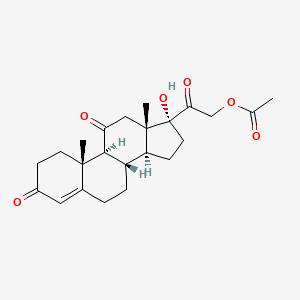

1. 17-hydroxy-3,11,20-trioxopregn-4-en-21-yl Acetate
2. Adreson
3. Cortisone
4. Cortone Acetate
1. Cortisone 21-acetate
2. 50-04-4
3. Cortone Acetate
4. Incortin
5. Biocort Acetate
6. Artriona
7. Cortadren
8. Scheroson
9. Cortisyl
10. Adreson
11. Irisone Acetate
12. Cortelan
13. Cortistab
14. Ricortex
15. Cortogen Acetate
16. Compound E Acetate
17. Pregn-4-ene-3,11,20-trione, 21-(acetyloxy)-17-hydroxy-
18. Cortisone Monoacetate
19. Nsc 49420
20. Cortisone, 21-acetate
21. Cortilen
22. Cortisone Aceticum
23. 11-dehydro-17-hydroxycorticosterone-21-acetate
24. 11-dehydro-17-hydroxycorticosterone Acetate
25. 17,21-dihydroxypregn-4-ene-3,11,20-trione 21-acetate
26. Nsc-49420
27. 883wkn7w8x
28. Chebi:3897
29. Pregn-4-ene-3,11,20-trione, 17,21-dihydroxy-, 21-acetate
30. Cortisone (acetate)
31. Ncgc00021191-07
32. 17-hydroxy-3,11,20-trioxopregn-4-en-21-yl Acetate
33. Dsstox_cid_2858
34. Dsstox_rid_76761
35. Dsstox_gsid_22858
36. 17,21-dihydroxypregn-4-ene-3,11,20-trione-21-acetate
37. 17alpha,21-dihydroxypregn-4-ene-3,11,20-trione Acetate
38. Cas-50-04-4
39. [2-[(8s,9s,10r,13s,14s,17r)-17-hydroxy-10,13-dimethyl-3,11-dioxo-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
40. 2-((8s,9s,10r,13s,14s,17r)-17-hydroxy-10,13-dimethyl-3,11-dioxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl Acetate
41. Smr000059124
42. Ccris 3661
43. Cortisone Acetate (cortone)
44. Einecs 200-006-5
45. Unii-883wkn7w8x
46. Cortisoneacetate
47. Cortisone Acetate [usp:jan]
48. Acetate Cortisone
49. Cortisyl Artriona
50. Component Of Neosone
51. Mfcd00003609
52. Neosone (salt/mix)
53. 21-(acetyloxy)-17-hydroxypregn-4-ene-3,11,20-trione
54. Cortisone-21-acetate
55. 21-acetoxy-17,alpha-hydroxy-3,11,20-triketopregnene-4
56. 21-acetoxy-17,alpha-hydroxypregn-4-ene-3,11,20-trione
57. Cortone Acetate (tn)
58. 4-pregnene-17,alpha,21-diol-3,11,20-trione 21-acetate
59. Schembl4501
60. Chembl1650
61. Lopac0_000271
62. Mls000069478
63. Mls002207136
64. Mls002548859
65. 21-acetoxy-4-pregnen-17alpha-ol-3,11,20-trione
66. Cortisone Acetate [jan]
67. Cortisone Acetate (jp17/usp)
68. Dtxsid0022858
69. Cortisone 21-acetate, >=99%
70. Cortisone Acetate [vandf]
71. Cortisone Aceticum [hpus]
72. Cortisone Acetate [mart.]
73. 17alpha,21-dihydroxy-4-pregnene-3,11,20-trione 21-acetate
74. Hms2235d24
75. Hms3259n19
76. Hms3260h04
77. Cortisone Acetate [usp-rs]
78. Cortisone Acetate [who-dd]
79. Bcp08487
80. Cortisone 21-acetate [mi]
81. Nsc49420
82. Zinc3875334
83. Tox21_113482
84. Tox21_500271
85. Bdbm50455157
86. Hy-17461a
87. Lmst02030120
88. Akos005267225
89. Tox21_113482_1
90. Ac-6827
91. Ccg-204366
92. Cortisone Acetate [orange Book]
93. Cs-1742
94. Db01380
95. Lp00271
96. Nc00677
97. Sdccgsbi-0050259.p002
98. Cortisone Acetate [ep Monograph]
99. Cortisone Acetate [usp Monograph]
100. Ncgc00021191-03
101. Ncgc00021191-06
102. Ncgc00021191-08
103. Ncgc00021191-09
104. Ncgc00021191-16
105. Ncgc00260956-01
106. [2-[(8s,9s,10r,13s,14s,17r)-17-hydroxy-10,13-dimethyl-3,11-dioxo-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]-2-oxo-ethyl] Acetate
107. 2-((1s,10s,11s,15s,2r,14r)-14-hydroxy-2,15-dimethyl-5,17-dioxotetracyclo[8.7.0 .0<2,7>.0<11,15>]heptadec-6-en-14-yl)-2-oxoethyl Acetate
108. As-13692
109. Cortisone Acetate 100 Microg/ml In Methanol
110. Eu-0100271
111. S2559
112. C08173
113. C90609
114. D00973
115. Ab00384283-08
116. Ab00384283_09
117. Cortisone Acetate 100 Microg/ml In Acetonitrile
118. 003c609
119. W-105993
120. Brd-k86161929-001-13-3
121. Hydrocortisone Acetate Impurity D [ep Impurity]
122. Pregn-4-ene-3,20-trione, 21-(acetyloxy)-17-hydroxy-
123. 17-hydroxy-3,11,20-trioxopregn-4-en-21-yl Acetate #
124. 21-acetoxy-17.alpha.-hydroxy-3,11,20-triketopregnene-4
125. 21-acetoxy-17.alpha.-hydroxypregn-4-ene-3,11,20-trione
126. 4-pregnen-17.alpha.,21-diol-3,11,20-trione 21-acetate
127. Pregn-4-ene-3,20-trione, 17,21-dihydroxy-, 21-acetate
128. 4-pregnene-17.alpha.,21-diol-3,11,20-trione 21-acetate
129. Cortisone Acetate, European Pharmacopoeia (ep) Reference Standard
130. Cortisone Acetate, United States Pharmacopeia (usp) Reference Standard
131. Cortisone Acetate, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 402.5 g/mol |
|---|---|
| Molecular Formula | C23H30O6 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 402.20423867 g/mol |
| Monoisotopic Mass | 402.20423867 g/mol |
| Topological Polar Surface Area | 97.7 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 827 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cortisone acetate |
| PubMed Health | Cortisone Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Drug Label | Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract.Cortisone acetate is a white or practically white, odorless, crystalline powder. It is stable in air. It i... |
| Active Ingredient | Cortisone acetate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Hikma Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Cortisone acetate |
| PubMed Health | Cortisone Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Drug Label | Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract.Cortisone acetate is a white or practically white, odorless, crystalline powder. It is stable in air. It i... |
| Active Ingredient | Cortisone acetate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Hikma Pharms |
Cortisone acetate is indicated to treat a wide variety of endocrine, rheumatic, collagen, dermatologic, allergic, ophthalmic, respiratory, hematologic, neoplastic, edematous, and gastrointestinal diseases and disorders.
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. The duration of action is moderate as it is generally given once daily. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Route of Elimination
Corticosteroids are eliminated predominantly in the urine.
Clearance
Data regarding the clearance of cortisone acetate is not readily available.
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.