
1. Copper(i) Acetate
2. Copper(ii) Acetate
3. Copper(ii) Acetate Monohydrate
4. Cu(ii) Acetate
5. Hoganite
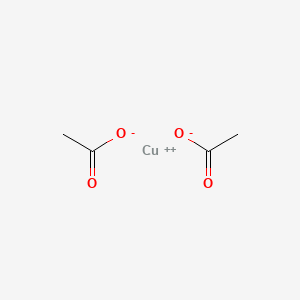
1. Copper(ii) Acetate
2. 142-71-2
3. Copper Diacetate
4. Copper Acetate
5. Copper(ii)acetate
6. Copper(2+) Acetate
7. Copper(2+) Diacetate
8. Copper (ii) Acetate
9. Cupric Diacetate
10. Neutral Verdigris
11. Crystals Of Venus
12. Acetic Acid, Copper(2+) Salt
13. Copper Acetate (cu(c2h3o2)2)
14. Crystallized Verdigris
15. Acetic Acid, Copper(2+) Salt, Basic
16. Cupric Acetate,anhydrous
17. Copper Acetate (cu(oac)2)
18. Acetic Acid Copper(2+) Salt
19. 39m11xph03
20. Acetic Acid, Copper(ii) Salt (2:1)
21. 52503-64-7
22. Copper Di(acetate)
23. Acetic Acid, Copper(2+) Salt (2:1)
24. Venus Copper
25. Copper(ii)-acetate
26. Acetic Acid, Copper Salt
27. Octan Mednaty [czech]
28. Octan Mednaty
29. Acetic Acid, Cupric Salt
30. Mfcd00008690
31. Acetate De Cuivre
32. Acetate De Cuivre [french]
33. Cu(ii) Acetate
34. Ccris 5286
35. Hsdb 915
36. Cupric Acetate, Basic
37. Nsc-75796
38. 4180-12-5
39. Einecs 205-553-3
40. Nsc 75796
41. Diacetoxycopper
42. Bisacetoxycopper
43. Unii-39m11xph03
44. Copper;diacetate
45. Diacetoxy Copper
46. Ai3-01379
47. Acetic Acid, Copper (2+) Salt
48. Copper Acetate Salt
49. Copper(ii)diacetate
50. Copper (ii)acetate
51. Copper-(ii)acetate
52. Einecs 257-974-7
53. Copper(1i) Acetate
54. Copper(11) Acetate
55. Copper(ii) Diacetate
56. Copper-(ii) Acetate
57. Copper-(ii)-acetate
58. Cuprum Aceticum
59. Copper (ii) Diacetate
60. Diacetoxy Copper (ii)
61. Acetic Acid,copper Salt
62. Cu(oac)2
63. Ec 205-553-3
64. Schembl1080
65. Cupric Acetate [mi]
66. Acetic Acid Copper(ii) Salt
67. Copper,bis(acetato-ko,ko')-
68. Cupric Acetate [inci]
69. Cuprum Aceticum [hpus]
70. Copper Acetate [mart.]
71. Copper Acetate [who-dd]
72. Dtxsid2059720
73. Copper(ii) Acetate [hsdb]
74. Copper(ii) Acetate, Anhydrous
75. Akos015892699
76. Copper(ii) Acetate, Trace Metals Grade
77. Ft-0653868
78. D77909
79. Q421854
80. J-520114
| Molecular Weight | 181.63 g/mol |
|---|---|
| Molecular Formula | C4H6CuO4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 180.956206 g/mol |
| Monoisotopic Mass | 180.956206 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Medication (VET): In hematinic mixtures (liquid or tablets) as source of copper.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 132
There are limited data concerning toxicity of copper in experimental animals. Heavy accumulation of copper was observed in the liver and kidneys of rats maintained for 16 mo on a diet supplemented with 5,000 ppm copper acetate. Similar depositions were seen in the liver and kidney as well as the brain and the large and small bowel in rats exposed to 1250 ppm cupric acetate monohydrate in the drinking water for up to 902 days.
USEPA; Health Issue Assessment: Copper p.33 (1987) EPA/600/8-87/001
The effects of cadmium and copper on the sodium ion (Na(+)) glucose cotransport in the kidney cortical cells of 4 week old male C57B16-rats were studied. Different concentrations of cadmium chloride, zinc chloride, or cupric acetate were added to the culture media. The Na(+)-glucose cotransport was estimated from the uptake of radiolabeled alpha-methylglucoside using scintillation counting techniques. Metallothionein was measured by Sephadex-G-75 column chromatography and subsequent high performance liquid chromatography. For Cd(2+) and Cu(2+), an increase in concentration resulted in an increase in the extent of inhibition of the Na(+)-glucose cotransport with approximately 3 hour lag times for the induction of inhibition. Increases in metal ion concentration also resulted in slower increases of metallothionein protein, with the rapid induction of metallothionein messenger RNA after incubation with the ions. Pretreatment of the cells with zinc(2+) reduced the effects of Cd(2+) and Cu(2+) on the cotransport by delaying the onset and reducing the extent of the inhibition.
PMID:7992308 Blumenthal S et al; Toxicology and Applied Pharmacology 129 (2): 177-87 (1994)
Ascorbic acid incubation reduced the occurrence of copper acetate induced increases in methemoglobin, while not effecting changes in glutathione in rats.
PMID:6635265 Calabrese EJ et al; Regul Toxicol Pharmacol 3 (3): 179-83 (1983)
