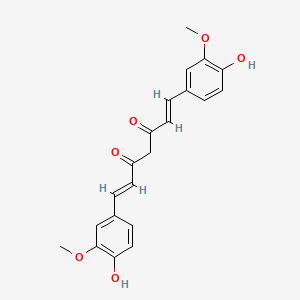

1. 1,6-heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (e,e)-
2. Curcumin Phytosome
3. Diferuloylmethane
4. Mervia
5. Phytosome, Curcumin
6. Turmeric Yellow
7. Yellow, Turmeric
1. 458-37-7
2. Diferuloylmethane
3. Turmeric Yellow
4. Natural Yellow 3
5. Turmeric
6. Indian Saffron
7. Kacha Haldi
8. Curcuma
9. Gelbwurz
10. Haldar
11. Merita Earth
12. Curcumin I
13. (1e,6e)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione
14. Souchet
15. Haidr
16. Halad
17. Halud
18. Terra Merita
19. Yellow Ginger
20. Yellow Root
21. Safran D'inde
22. C.i. Natural Yellow 3
23. Yo-kin
24. Curcuma Oil
25. Golden Seal
26. Orange Root
27. Curcumine
28. Hydrastis
29. Indian Turmeric
30. Yellow Puccoon
31. Diferaloylmethane
32. Turmeric Oleoresin
33. Kurkumin [czech]
34. Tumeric Yellow
35. Ci Natural Yellow 3
36. C.i. 75300
37. Zlut Prirodni 3 [czech]
38. Cucurmin
39. Curcumin (synthetic)
40. 8024-37-1
41. Tumeric Oleoresin
42. E 100
43. Curcurmin
44. Turmeric Oleoresin (79%-85% Curcumin)
45. Ci 75300
46. 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
47. Nsc32982
48. 94875-80-6
49. Mfcd00008365
50. Nsc 32982
51. Turmeric (>98% Curcurmin)
52. Chebi:3962
53. Mls000069631
54. Turmeric Extract
55. 1,6-heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (e,e)-
56. Nsc-32982
57. 1,9-bis(4-hydroxy-3-methoxyphenyl)-2,7-nonadiene-4,6-dione
58. Chembl140
59. Oils, Curcuma
60. 1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione
61. (1e,6e)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
62. Smr000058237
63. 1,6-heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (1e,6e)-
64. It942zth98
65. Turmeric Oil
66. Oil Of Turmeric
67. (e,e)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
68. Nsc687842
69. Curcuma Longa Oils
70. Nsc-687842
71. Ncgc00017159-05
72. Kurkumin
73. Dsstox_cid_1421
74. (1e,6e)-1,7-bis(4-hydroxy-3-methoxy-phenyl)hepta-1,6-diene-3,5-dione
75. (1e,6e)-1,7-bis[4-hydroxy-3-(methyloxy)phenyl]hepta-1,6-diene-3,5-dione
76. Zlut Prirodni 3
77. Turmeric Root Oil
78. Dsstox_rid_78861
79. Dsstox_gsid_31077
80. Turmeric, Oleoresin
81. Curcuma Oil (curcuma Longa)
82. Turmeric Oil (curcuma Longa L.)
83. Curcuma Longa L. Root Oil
84. Cas-458-37-7
85. Fema No. 3085
86. Fema No. 3086
87. Ccris 3257
88. Ccris 5804
89. Hsdb 4334
90. 1,5-di(vanillyliden)acetylaceton
91. Nci-c61325
92. Sr-01000000149
93. 1,5-divanillyliden-2,4-pentandion
94. Einecs 207-280-5
95. Nsc 687842
96. Brn 2306965
97. Unii-it942zth98
98. E 100 (dye)
99. Curcumin Solution
100. Turmeric; Curcuma
101. Curcumin,(s)
102. (e/z)-curcumin
103. Trans,trans-curcumin
104. Starbld0017234
105. Curcumin [hsdb]
106. Curcumin [inci]
107. Curcumin [mi]
108. Opera_id_1627
109. Curcumin [mart.]
110. Curcumin [usp-rs]
111. Curcumin [who-dd]
112. Schembl8440
113. Schembl8441
114. Curcumin, Analytical Standard
115. 4-08-00-03697 (beilstein Handbook Reference)
116. Mls001148449
117. Bidd:er0479
118. Cu-01000001305-2
119. Turmeric Root Oil Co2 Extract
120. Cid_969516
121. Gtpl7000
122. Turmeric Root Oil Hydrodistilled
123. Dtxsid8031077
124. Schembl13521974
125. Bdbm29532
126. Cid_5281767
127. Cmap_000052
128. Ci 75300 [inci]
129. Hms2233k04
130. Hms3649k06
131. Zinc899824
132. 2,7-nonadiene-4,6-dione, 1,9-bis(4-hydroxy-3-methoxyphenyl)-
133. 91884-86-5
134. Amy33436
135. Bcp04695
136. Turmeric (>98% Curcumin)
137. Tox21_110803
138. Tox21_111505
139. Tox21_201116
140. Bbl027711
141. Bdbm50067040
142. Bdbm50140172
143. Ccg-36020
144. Ccg-36107
145. Stl371943
146. Akos001305497
147. Bcp9000557
148. Curcuma Longa L. Root Oil Co2 Extract
149. Db11672
150. Curcumin 100 Microg/ml In Acetonitrile
151. Curcuma Longa L. Root Oil Hydrodistilled
152. Ncgc00017159-04
153. Ncgc00017159-06
154. Ncgc00017159-07
155. Ncgc00017159-09
156. Ncgc00017159-10
157. Ncgc00017159-11
158. Ncgc00017159-12
159. Ncgc00023332-03
160. Ncgc00023332-04
161. Ncgc00023332-05
162. Ncgc00258668-01
163. Ac-24238
164. As-72202
165. Bp-25396
166. Bcp0726000035
167. Db-002681
168. Wln: 1or Bq E1u1v1v1u1r Dq Co1
169. C-230
170. Cs-0149275
171. N1839
172. 1,3-di(3-methoxy-4-hydroxystyryl)propanedial
173. F21478
174. K00009
175. Curcumin (constituent Of Turmeric) [dsc]
176. Curcumin, Curcuma Longa L. - Cas 458-37-7
177. Curcumin, From Curcuma Longa (turmeric), Powder
178. 458c377
179. A826902
180. Curcumin, Primary Pharmaceutical Reference Standard
181. Q312266
182. 1,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-
183. Sr-01000000149-2
184. Sr-01000000149-5
185. Brd-k07572174-001-02-2
186. Brd-k07572174-001-19-6
187. Brd-k07572174-001-22-0
188. Curcumin, >=94% (curcuminoid Content), >=80% (curcumin)
189. 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione
190. 1,7-bis(4-hydroxy-3-methoxyphenyl)1,6-heptadiene-3,5-dione
191. Curcumin, (total Curcuminoid Content), From Turmeric Rhizome
192. Curcumin, Matrix Substance For Maldi-ms, >=99.5% (hplc)
193. Curcumin, United States Pharmacopeia (usp) Reference Standard
194. 1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione
195. 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
196. ((e,e)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione)
197. (1e,6e)-1,7-bis(3-methoxy-4-oxidanyl-phenyl)hepta-1,6-diene-3,5-dione
198. (1e,6e)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione #
199. (1e,6e)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione.
200. (1e,6e)-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione
201. (1z,6e)-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione
202. 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, (e,e)-
203. 1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione
204. 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one
205. 5-hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one
206. Curcumin; 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
207. (1e,4z,6e)-5-hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one
208. Curcumin Solution, ~0.1 % (w/v) (in Ethanol With 2m Hcl (99:1 V/v)), For Tlc Derivatization
| Molecular Weight | 368.4 g/mol |
|---|---|
| Molecular Formula | C21H20O6 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 368.12598835 g/mol |
| Monoisotopic Mass | 368.12598835 g/mol |
| Topological Polar Surface Area | 93.1 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 507 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPL THER Curcumin (Cum) has been reported to have potential chemo-preventive and chemotherapeutic activity through influencing various processes, inducing cell cycle arrest, differentiation and apoptosis in a series of cancers. However, the poor solubility of Cum limits its further applications in the treatment of cancer. /The authors/ have previously reported Cum-loaded nanoparticles (Cum-NPs) prepared with amphilic methoxy poly(ethylene glycol)-polycaprolactone (mPEG-PCL) block copolymers. The current study demonstrated superior antitumor efficacy of Cum-NPs over free Cum in the treatment of lung cancer. In vivo evaluation further demonstrated superior anticancer effects of Cum-NPs by delaying tumor growth compared to free Cum in an established A549 transplanted mice model. Moreover, Cum-NPs showed little toxicity to normal tissues including bone marrow, liver and kidney at a therapeutic dose. These results suggest that Cum-NPs are effective to inhibit the growth of human lung cancer with little toxicity to normal tissues, and could provide a clinically useful therapeutic regimen. They thus merit more research to evaluate the feasibility of clinical application./ Curcumin-loaded nanoparticles/
PMID:23534763 Yin HT et al; Asian Pac J Cancer Prev 14 (1): 409-12 (2013)
EXPL THER Accumulation of amyloid peptide (Abeta) in senile plaques is a hallmark lesion of Alzheimer disease (AD). The design of molecules able to target the amyloid pathology in tissue is receiving increasing attention, both for diagnostic and for therapeutic purposes. Curcumin is a fluorescent molecule with high affinity for the Abeta peptide but its low solubility limits its clinical use. Curcumin-conjugated nanoliposomes, with curcumin exposed at the surface, were designed. They appeared to be monodisperse and stable. They were non-toxic in vitro, down-regulated the secretion of amyloid peptide and partially prevented Abeta -induced toxicity. They strongly labeled Abeta deposits in post-mortem brain tissue of AD patients and APPxPS1 mice. Injection in the hippocampus and in the neocortex of these mice showed that curcumin-conjugated nanoliposomes were able to specifically stain the Abeta deposits in vivo. Curcumin-conjugated nanoliposomes could find application in the diagnosis and targeted drug delivery in AD. In this preclinical study, curcumin-conjugated nanoliposomes were investigated as possible diagnostics and targeted drug delivery system in Alzheimer's disease, demonstrating strong labeling of Abeta deposits both in human tissue and in mice, and in vitro downregulation of amyloid peptide secretion and prevention of Abeta -induced toxicity./ Curcumin-conjugated nanoliposomes/
PMID:23220328 Lazar AN et al; Nanomedicine 9 (5): 712-21 (2013)
EXPL THER The anti-inflammatory agent curcumin can selectively eliminate malignant rather than normal cells. The present study examined the effects of curcumin on the Lewis lung carcinoma (LLC) cell line and characterized a subpopulation surviving curcumin treatments. Cell density was measured after curcumin was applied at concentrations between 10 and 60 uM for 30 hours. Because of the high cell loss at 60 uM, this dose was chosen to select for surviving cells that were then used to establish a new cell line. The resulting line had approximately 20% slower growth than the original LLC cell line and based on ELISA contained less of two markers, NF-kB and ALDH1A, used to identify more aggressive cancer cells. /The authors/ also injected cells from the original and surviving lines subcutaneously into syngeneic C57BL/6 mice and monitored tumor development over three weeks and found that the curcumin surviving-line remained tumorigenic. Because curcumin has been reported to kill cancer cells more effectively when administered with light, /the authors/ examined this as a possible way of enhancing the efficacy of curcumin against LLC cells. When LLC cells were exposed to curcumin and light from a fluorescent lamp source, cell loss caused by 20 uM curcumin was enhanced by about 50%, supporting a therapeutic use of curcumin in combination with white light. This study is the first to characterize a curcumin-surviving subpopulation among lung cancer cells. It shows that curcumin at a high concentration either selects for an intrinsically less aggressive cell subpopulation or generates these cells. The findings further support a role for curcumin as an adjunct to traditional chemical or radiation therapy of lung and other cancers.
PMID:22232696 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3253430 Yan D et al; J Cancer 3: 32-41 (2012)
EXPL THER 5-Fluorouracil (5-FU) is the first rationally designed antimetabolite, which achieves its therapeutic efficacy through inhibition of the enzyme thymidylate synthase (TS), which is essential for the synthesis and repair of DNA. However, prolonged exposure to 5-FU induces TS overexpression, which leads to 5-FU resistance in cancer cells. Several studies have identified curcumin as a potent chemosensitizer against chemoresistance induced by various chemotherapeutic drugs. In this study, /investigators/ report for the first time, with mechanism-based evidences, that curcumin can effectively chemosensitize breast cancer cells to 5-FU, thereby reducing the toxicity and drug resistance. /The authors/ found that 10 uM 5-FU and 10 uM curcumin induces a synergistic cytotoxic effect in different breast cancer cells, independent of their receptor status, through the enhancement of apoptosis. Curcumin was found to sensitize the breast cancer cells to 5-FU through TS-dependent downregulation of nuclear factor-kB (NF-kB), and this observation was confirmed by silencing TS and inactivating NF-kB, both of which reduced the chemosensitizing efficacy of curcumin. Silencing of TS suppressed 5-FU-induced NF-kB activation, whereas inactivation of NF-kB did not affect 5-FU-induced TS upregulation, confirming that TS is upstream of NF-kB and regulates the activation of NF-kB in 5-FU-induced signaling pathway. Although Akt/PI3kinase and mitogen-activated protein kinase pathways are activated by 5-FU and downregulated by curcumin, they do not have any role in regulating the synergism. As curcumin is a pharmacologically safe and cost-effective compound, its use in combination with 5-FU may improve the therapeutic index of 5-FU, if corroborated by in vivo studies and clinical trials.
PMID:23429291 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3734809 Vinod BS et al; Cell Death Dis 4: e505 (2013)
For more Therapeutic Uses (Complete) data for CURCUMIN (23 total), please visit the HSDB record page.
No approved therapeutic indications.
Intravenous application of 25 mg/kg bw curcumin to rats resulted in an increase in bile flow by 80 and 120%. In the rat model of inflammation, curcumin was shown to inhibit edema formation. In nude mouse that had been injected subcutaneously with prostate cancer cells, administration of curcumin caused a marked decrease in the extent of cell proliferation, a significant increase of apoptosis and micro-vessel density. Curcumin may exert choleretic effects by increasing biliary excretion of bile salts, cholesterol, and bilirubin, as well as increasing bile solubility. Curcumin inhibited arachidonic acid-induced platelet aggregation _in vitro_.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Absorption
Curcumin displays poor absorption into the gastrointestinal tract. In a rat study, oral administration of a single dose of 2 g of curcumin resulted in a plasma concentration of less than 5 g/mL, indicating poor absorption from the gut.
Route of Elimination
Following oral administration of curcumin to rats at a dose of 1 g/kg bw, about 75% of dose was excreted in the faeces and only traces of the compound was detected in the urine. When a single 400 mg dose of curcumin was administered orally to rats, about 60% was absorbed and 40% was excreted unchanged in the faeces over an period of 5 days. Intraperitoneal administration resulted in fecal excretion of 73% and biliary excretion of 11%.
Volume of Distribution
Following oral administration of radio-labelled curcumin to rats, radioactivity was detected in the liver and kidneys.
Clearance
No pharmacokinetic data available.
Oral & ip doses of (3)H-curcumin led to fecal excretion of most of radioactivity. Iv & ip doses were well excreted in bile of cannulated rats.
HOLDER ET AL; XENOBIOTICA 8(12) 761 (1978)
When admin orally in dose of 1 g/kg, curcumin was excreted in feces to about 75%, while negligible amt appeared in urine. Measurement of blood plasma levels & biliary excretion showed that curcumin was poorly absorbed from the gut.
WAHLSTROM B, BLENNOW G; ACTA PHARMACOL TOXICOL 43(2) 86 (1978)
The aim of this study was to develop a curcumin intranasal thermosensitive hydrogel and to improve its brain targeting efficiency. The hydrogel gelation temperature, gelation time, drug release and mucociliary toxicity characteristics as well as the nose-to-brain transport in the rat model were evaluated. The developed nasal hydrogel, composed of Pluronic F127 and Poloxamer 188, had shorter gelation time, longer mucociliary transport time and produced prolonged curcumin retention in the rat nasal cavity at body temperature. The hydrogel release mechanism was diffusion-controlled drug release, evaluated by the dialysis membrane method, but dissolution-controlled release when evaluated by the membraneless method. A mucociliary toxicity study revealed that the hydrogel maintained nasal mucosal integrity until 14 days after application. The drug-targeting efficiencies for the drug in the cerebrum, cerebellum, hippocampus and olfactory bulb after intranasal administration of the curcumin hydrogel were 1.82, 2.05, 2.07 and 1.51 times that after intravenous administration of the curcumin solution injection, respectively, indicating that the hydrogel significantly increased the distribution of curcumin into the rat brain tissue, especially into the cerebellum and hippocampus. A thermosensitive curcumin nasal gel was developed with favourable gelation, release properties, biological safety and enhanced brain-uptake efficiency. /Curcumin intranasal thermosensitive hydrogel/
PMID:23647674 Chen X et al; J Pharm Pharmacol 65 (6): 807-16 (2013)
...However, curcumin has a low systemic bioavailability, so it is imperative to improve the bioavailability of curcumin in its clinical application. Many methods, such as adjuvant drug delivery system and structural modification have been demonstrated to have a potential effect.
PMID:23116310 Fan X et al; Curr Pharm Des 19 (11): 2011-31 (2013)
For more Absorption, Distribution and Excretion (Complete) data for CURCUMIN (6 total), please visit the HSDB record page.
Initially, curcumin undergoes rapid intestinal metabolism to form curcumin glucuronide and curcumin sulfate via O-conjugation. Other metabolites formed include tetrahydrocurcumin, hexahydrocurcumin, and hexahydrocurcuminol via reduction. Curcumin may also undergo intensive second metabolism in the liver where the major metabolites were glucuronides of tetrahydrocurcumin and hexahydrocurcumin, with dihydroferulic acid and traces of ferulic acid as further metabolites. Hepatic metabolites are expected to be excreted in the bile. Certain curcumin metabolites, such as tetrahydrocurcumin, retain anti-inflammatory and antioxidant properties.
Iv & ip doses of (3)H-curcumin excreted in bile of cannulated rats. Major metab were glucuronides of tetrahydrocurcumin & hexahydrocurcumin. Minor metab was dihydroferulic acid together with traces of ferulic acid.
HOLDER ET AL; XENOBIOTICA 8(12) 761 (1978)
Curcumin has known human metabolites that include Curcumin 4-O-glucuronide and O-demethyl curcumin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
No pharmacokinetic data available.
Curcumin acts as a scavenger of oxygen species, such as hydroxyl radical, superoxide anion, and singlet oxygen and inhibit lipid peroxidation as well as peroxide-induced DNA damage. Curcumin mediates potent anti-inflammatory agent and anti-carcinogenic actions via modulating various signalling molecules. It suppresses a number of key elements in cellular signal transduction pathways pertinent to growth, differentiation, and malignant transformation; it was demonstrated _in vitro_ that curcumin inhibits protein kinases, c-Jun/AP-1 activation, prostaglandin biosynthesis, and the activity and expression of the enzyme cyclooxygenase (COX)-2.
Curcumin, a polyphenolic natural product, exhibits therapeutic activity against a number of diseases, attributed mainly to its chemical structure and unique physical, chemical, and biological properties. It is a diferuloyl methane molecule [1,7-bis (4-hydroxy-3- methoxyphenyl)-1,6-heptadiene-3,5-dione)] containing two ferulic acid residues joined by a methylene bridge. It has three important functionalities: an aromatic o-methoxy phenolic group, alpha, beta-unsaturated beta-diketo moiety and a seven carbon linker. Extensive research in the last two decades has provided evidence for the role of these different functional groups in its crucial biological activities. A few highlights of chemical structural features associated with the biological activity of curcumin are: The o-methoxyphenol group and methylenic hydrogen are responsible for the antioxidant activity of curcumin, and curcumin donates an electron/ hydrogen atom to reactive oxygen species. Curcumin interacts with a number of biomolecules through non-covalent and covalent binding. The hydrogen bonding and hydrophobicity of curcumin, arising from the aromatic and tautomeric structures along with the flexibility of the linker group are responsible for the non-covalent interactions. The alpha, beta-unsaturated beta-diketone moiety covalently interacts with protein thiols, through Michael reaction. The beta-diketo group forms chelates with transition metals, thereby reducing the metal induced toxicity and some of the metal complexes exhibit improved antioxidant activity as enzyme mimics. New analogues with improved activity are being developed with modifications on specific functional groups of curcumin...
PMID:23116315 Priyadarsini KI; Curr Pharm Des 19 (11): 2093-100 (2013)
The present study demonstrates that curcumin acts as pro-oxidant and sensitizes human lung adenocarcinoma epithelial cells (A549) to apoptosis via intracellular redox status mediated pathway. Results indicated that curcumin induced cell toxicity (light microscopy and MTT assay) and apoptosis (AnnexinV-FITC/PI labeling and caspase-3 activity) in these cells. These events seem to be mediated through generation of reactive oxygen species (ROS) and superoxide radicals (SOR) and enhanced levels of lipid peroxidation. These changes were accompanied by increase in oxidized glutathione (GSSG), reduced glutathione (GSH) and gamma-glutamylcysteine synthetase (gamma-GCS) activity, but decrease in GSH/GSSG ratio. The induction of apoptosis and decrease in GSH/GSSG ratio was also accompanied by sustained phosphorylation and activation of p38 mitogen activated protein kinase (MAPK). On the other hand, addition of N-acetyl cysteine (NAC), an antioxidant, blocked the curcumin-induced ROS production and rescued malignant cells from curcumin-induced apoptosis through caspase-3 deactivation. However, L-buthionine sulfoximine (BSO), a GSH synthesis blocking agent, further enhanced curcumin-induced ROS production and apoptosis in A549 cells. Decreased GSH/GSSG ratio seems to be a crucial factor for the activation of MAPK signaling cascade by curcumin. The study therefore, provides an insight into the molecular mechanism involved in sensitization of lung adenocarcinoma cells to apoptosis by curcumin.
PMID:23986968 Kaushik G et al; Indian J Exp Biol 50 (12): 853-61 (2012)
Curcumin has many pharmaceutical applications, many of which arise from its potent antioxidant properties. The present research examined the antioxidant activities of curcumin in polar solvents by a comparative study using ESR, reduction of ferric iron in aqueous medium and intracellular ROS/toxicity assays. ESR data indicated that the steric hindrance among adjacent big size groups within a galvinoxyl molecule limited the curcumin to scavenge galvinoxyl radicals effectively, while curcumin showed a powerful capacity for scavenging intracellular smaller oxidative molecules such as H2O2, HO-, ROO-. Cell viability and ROS assays demonstrated that curcumin was able to penetrate into the polar medium inside the cells and to protect them against the highly toxic and lethal effects of cumene hydroperoxide. Curcumin also showed good electron-transfer capability, with greater activity than trolox in aqueous solution. Curcumin can readily transfer electron or easily donate H-atom from two phenolic sites to scavenge free radicals. The excellent electron transfer capability of curcumin is because of its unique structure and different functional groups, including a beta-diketone and several pi electrons that have the capacity to conjugate between two phenyl rings. Therefore, since curcumin is inherently a lipophilic compound, because of its superb intracellular ROS scavenging activity, it can be used as an effective antioxidant for ROS protection within the polar cytoplasm.
PMID:22016801 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3189944 Barzegar A, Moosavi-Movahedi AA; PLoS One 6 (10): e26012 (2011)
Curcumin (diferuloylmethane), the yellow pigment in Indian saffron (Curcuma longa; also called turmeric, haldi, or haridara in the East and curry powder in the West), has been consumed by people for centuries as a dietary component and for a variety of proinflammatory ailments. Extensive research within the last decade in cell culture and in rodents has revealed that curcumin can sensitize tumors to different chemotherapeutic agents including doxorubicin, 5-FU, paclitaxel, vincristine, melphalan, butyrate, cisplatin, celecoxib, vinorelbine, gemcitabine, oxaliplatin, etoposide, sulfinosine, thalidomide, and bortezomib. Chemosensitization has been observed in cancers of the breast, colon, pancreas, gastric, liver, blood, lung, prostate, bladder, cervix, ovary, head and neck, and brain and in multiple myeloma, leukemia, and lymphoma. Similar studies have also revealed that this agent can sensitize a variety of tumors to gamma radiation including glioma, neuroblastoma, cervical carcinoma, epidermal carcinoma, prostate cancer, and colon cancer. How curcumin acts as a chemosensitizer and radiosensitizer has also been studied extensively. For example, it downregulates various growth regulatory pathways and specific genetic targets including genes for NF-kappaB, STAT3, COX2, Akt, antiapoptotic proteins, growth factor receptors, and multidrug-resistance proteins. Although it acts as a chemosensitizer and radiosensitizer for tumors in some cases, curcumin has also been shown to protect normal organs such as liver, kidney, oral mucosa, and heart from chemotherapy and radiotherapy-induced toxicity. The protective effects of curcumin appear to be mediated through its ability to induce the activation of NRF2 and induce the expression of antioxidant enzymes (e.g., hemeoxygenase-1, glutathione peroxidase, modulatory subunit of gamma-glutamyl-cysteine ligase, and NAD(P)H:quinone oxidoreductase 1, increase glutathione (a product of the modulatory subunit of gamma-glutamyl-cysteine ligase), directly quench free radicals, and inhibit p300 HAT activity.
PMID:20924967 Goel A, Aggarwal BB; Nutr Cancer 62 (7): 919-30 (2010)
For more Mechanism of Action (Complete) data for CURCUMIN (6 total), please visit the HSDB record page.