


1. Aminocyclohexylpenicillin
2. Ciclacillin
3. Wy 4508
4. Wy-4508
5. Wy4508
1. Ciclacillin
2. Vastcillin
3. Ciclacillum
4. Bastcillin
5. 3485-14-1
6. Cyclapen
7. Aminocyclohexylpenicillin
8. Citosarin
9. Syngacillin
10. Ultracillin
11. Calthor
12. Vipicil
13. Wyvital
14. Cyclapen-w
15. Orfilina
16. Ciclacilline
17. Ciclacillinum
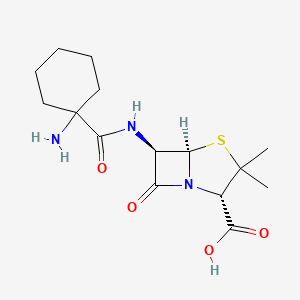
18. (1-aminocyclohexyl)penicillin
19. Ciclacilina
20. Ciclacilina [inn-spanish]
21. Ciclacilline [inn-french]
22. Ciclacillinum [inn-latin]
23. 6-(1-aminocyclohexanecarboxamido)penicillanic Acid
24. Aminocyclohexyl Penicillin
25. Wy-4508
26. Cyclacillin [usan]
27. 6-(1-aminocyclohexylcarboxamido)penicillanic Acid
28. Wy 4508
29. Ac 98
30. Penicillin, (aminocyclohexyl)-
31. Ciclacillin [inn]
32. Cyclacillin (usan)
33. (2s,5r,6r)-6-[(1-aminocyclohexanecarbonyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
34. Nsc88789
35. Nsc-88789
36. (2s,5r,6r)-6-{[(1-aminocyclohexyl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
37. Noblicil
38. Peamezin
39. Chebi:31444
40. 72zj154x86
41. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((1-aminocyclohexyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2s-(2alpha,5alpha,6beta))-
42. 6-(1-aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
43. Ncgc00016629-01
44. Cas-3485-14-1
45. 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid, 6-[[(1-aminocyclohexyl)carbonyl]amino]-3,3-dimethyl-7-oxo-, (2s,5r,6r)-
46. Ac-pc
47. Vastcillin (tn)
48. 6-(1-aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
49. Cyclapen-w (tn)
50. Mls002694856
51. Einecs 222-470-8
52. Nsc 88789
53. Wy4508
54. Cyclacillin [usan:usp]
55. Brn 0938663
56. Unii-72zj154x86
57. Cyclacillin [mi]
58. Prestwick0_001120
59. Prestwick1_001120
60. Prestwick2_001120
61. Prestwick3_001120
62. Ciclacillin [jan]
63. Dsstox_cid_2861
64. Ciclacillin (jp17/inn)
65. Cyclacillin [vandf]
66. Ciclacillin [mart.]
67. Dsstox_rid_76762
68. C-12104
69. Dsstox_gsid_22861
70. Schembl33900
71. Bspbio_001260
72. Ciclacillin [who-dd]
73. Spbio_003120
74. Bpbio1_001385
75. Gtpl4817
76. Chembl1200356
77. Dtxsid9022861
78. Cyclacillin [orange Book]
79. Hms1571o22
80. Zinc3830609
81. Tox21_110534
82. Bdbm50350474
83. 6beta-(1-aminocyclohexanecarboxamido)-2,2-dimethylpenam-3alpha-carboxylic Acid
84. Db01000
85. Ncgc00179238-01
86. (2s,5r,6r)-6-(1-aminocyclohexaneamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
87. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(1-aminocyclohexanecarboxamido)-3,3-dimethyl-7-oxo-
88. Hy-16158
89. Nci60_041965
90. Ab00514059
91. Cs-0006169
92. D01334
93. Q5119441
94. Brd-k89046952-001-03-4
95. Wln: T45 Anv Estj F1 F1 Gvq Cmv- Al6tj Az
96. (2s,5r,6r)-6-[(1-aminocyclohexane)amido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
| Molecular Weight | 341.4 g/mol |
|---|---|
| Molecular Formula | C15H23N3O4S |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 341.14092740 g/mol |
| Monoisotopic Mass | 341.14092740 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 559 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of bacterial infections caused by susceptible organisms.
Cyclacillin, a penicillin, is a cyclohexylamido analog of penicillanic acid. Cyclacillin is more resistant to beta-lactamase hydrolysis than ampicillin, is much better absorbed when given by mouth and, as a result, the levels reached in the blood and in the urine are considerably higher than those obtained with the same dose of ampicillin. Cyclacillin has been replaced by newer penicillin treatments.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Absorption
Moderately absorbed.
The bactericidal activity of cyclacillin results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). Cyclacillin is stable in the presence of a variety of b-lactamases, including penicillinases and some cephalosporinases.