
1. Actidione
2. Cicloheximide
1. 66-81-9
2. Actidione
3. Cicloheximide
4. Naramycin A
5. Kaken
6. Actidion
7. Actidone
8. Hizarocin
9. Naramycin
10. Neocycloheximide
11. Acti-aid
12. Cycloheximid
13. Actispray
14. Actidione Pm
15. Cicloheximida
16. Cicloheximidum
17. Zykloheximid
18. Nsc-185
19. Cycloheximide [usan]
20. C15h23no4
21. Nsc 185
22. U-4527
23. Acti-dione Br
24. 4-[(2r)-2-[(1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl]-2-hydroxyethyl]piperidine-2,6-dione
25. 4-{(2r)-2-[(1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl]-2-hydroxyethyl}piperidine-2,6-dione
26. Cbc 500046
27. 3-((r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)glutarimide
28. Nsc185
29. Cicloheximide [inn]
30. Acti-dione
31. Actidione Tgf
32. Tcmdc-125838
33. U 4527
34. Aktidion
35. Chembl123292
36. Tza
37. Chebi:27641
38. Gnf-pf-5118
39. Beta-(2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)glutarimide
40. 3-(2r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethylglutarimide
41. Cicloheximide (inn)
42. (1s-(1alpha(s*),3alpha,5beta))-4-(2-(3,5-dimethyl-2-oxo-cyclohexyl))-2-hydroxyethyl-2,6-piperidinedione
43. Cx
44. Cycloheximide (usan)
45. Actidione Br
46. Dsstox_cid_4882
47. 2,6-piperidinedione, 4-[(2r)-2-[(1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl]-2-hydroxyethyl]-
48. 4-((r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)piperidine-2,6-dione
49. 4-[(2r)-2-[(1s,3s,5s)-3,5-dimethyl-2-oxo-cyclohexyl]-2-hydroxy-ethyl]piperidine-2,6-dione
50. Aktidion [czech]
51. Dsstox_rid_77565
52. Cyclohemimide
53. Cyclohexamide
54. Dsstox_gsid_24882
55. 98600c0908
56. 3-(2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)glutarimide
57. 3-[2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]glutarimide
58. Caswell No. 270a
59. .beta.-[2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]glutarimide
60. 4-[2-(3,5-dimethyl-2-oxo-cyclohexyl)-2-hydroxyethyl]-2,6-piperidinedione
61. Cicloheximidum [inn-latin]
62. Cicloheximida [inn-spanish]
63. Smr000686067
64. Ccris 937
65. Hsdb 1552
66. Sr-01000597484
67. Sr-05000001596
68. Einecs 200-636-0
69. Cycloheximide [bsi:iso]
70. Epa Pesticide Chemical Code 043401
71. Brn 0088868
72. Nm-mcd 80
73. Ai3-15541
74. Dtxsid6024882
75. Acti-dione Pm
76. Acti-dione Tgf
77. Cas-66-81-9
78. Ncgc00024910-01
79. 2,6-piperidinedione, 4-((2r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)-
80. 4-((2r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)piperidine-2,6-dione
81. Prestwick_976
82. Mfcd00082346
83. Unii-98600c0908
84. 2,6-piperidinedione, 4-(2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)-, (1s-(1alpha(s*),3alpha,5beta))-
85. Spectrum_001344
86. Ft 3422-2
87. Prestwick0_000790
88. Prestwick1_000790
89. Prestwick2_000790
90. Prestwick3_000790
91. Spectrum2_000900
92. Spectrum3_001510
93. Spectrum4_000914
94. Spectrum5_001635
95. Cycloheximide [mi]
96. Cycloheximide [iso]
97. Cid_6197
98. 4-((2r)-2-((1s,3s,5s)-(3,5-dimethyl-2-oxocyclohexyl))-2-hydroxyethyl)piperidine-2,6-dione
99. Cycloheximide [hsdb]
100. Schembl26617
101. Bspbio_000900
102. Bspbio_003159
103. Kbiogr_001408
104. Kbioss_001824
105. 5-21-13-00434 (beilstein Handbook Reference)
106. Ksc-8-190
107. Mls001055333
108. Mls002154001
109. Cicloheximide [mart.]
110. Divk1c_000050
111. Spectrum1502112
112. Spbio_000720
113. Spbio_002839
114. 4-[2-(3,6-piperidinedione
115. Bpbio1_000990
116. Gtpl5433
117. Hms500c12
118. Kbio1_000050
119. Kbio2_001824
120. Kbio2_004392
121. Kbio2_006960
122. Kbio3_002659
123. Ninds_000050
124. Hms1570m22
125. Hms1921l04
126. Hms2092d10
127. Hms2097m22
128. Hms3714m22
129. Kuc105858n
130. Pharmakon1600-01502112
131. Cycloheximide, Streptomyces Griseus
132. 2,6-piperidinedione, 4-(2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)-, (1s-(1.alpha.(s*),3.alpha.,5.beta.))-
133. Zinc3872170
134. Tox21_113580
135. Tox21_201158
136. Tox21_303652
137. Bdbm50080528
138. Ccg-39902
139. Cycloheximide, >=93.0% (hplc)
140. Nsc758187
141. Akos024282670
142. Tox21_113580_1
143. Cs-4985
144. Nsc-758187
145. Idi1_000050
146. Ncgc00017363-05
147. Ncgc00017363-06
148. Ncgc00017363-07
149. Ncgc00017363-08
150. Ncgc00017363-10
151. Ncgc00017363-18
152. Ncgc00024910-02
153. Ncgc00024910-03
154. Ncgc00024910-04
155. Ncgc00024910-05
156. Ncgc00024910-06
157. Ncgc00169964-05
158. Ncgc00257430-01
159. Ncgc00258710-01
160. 4-[(2r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]azaperhydro Ine-2,6-dione
161. Ac-35130
162. As-14624
163. Bp-12396
164. Hy-12320
165. Nci60_001540
166. Sbi-0051732.p002
167. Wln: T6vmvtj E1yq- Bl6vtj D1 F1
168. Cycloheximide 100 Microg/ml In Acetonitrile
169. Cycloheximide, From Microbial, >=94% (tlc)
170. C-9217
171. C06685
172. Cycloheximide Solution, 0.1%, For Microbiology
173. D03625
174. M01527
175. Ab00052279_08
176. Cycloheximide, Pestanal(r), Analytical Standard
177. 082c346
178. Cycloheximide, Biotechnology Performance Certified
179. Q412895
180. Cycloheximide, Antibiotic For Culture Media Use Only
181. Sr-01000597484-1
182. Sr-01000597484-4
183. Sr-05000001596-1
184. Sr-05000001596-3
185. Sr-05000001596-4
186. Brd-k36055864-001-05-1
187. Brd-k36055864-001-07-7
188. Brd-k36055864-001-09-3
189. Brd-k36055864-001-16-8
190. [1s-[1.alpha.(s*),3.alpha.,5.beta.]]-4-[2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]-2,6-piperidinedione
191. 1154-57-0
192. 2, 4-[2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]-, [1s-[1.alpha.(s*),3.alpha.,5.beta.]]-
193. 4-((2r)-2-((1s,3s,5s)-3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl)-2,6-piperidinedione
194. 4-[(2r)-2-[(1s,3s,5r)-3,5-dimethyl-2-oxo-cyclohexyl]-2-hydroxy-ethyl]piperidine-2,6-dione
195. Cycloheximide Solution, Ready-made Solution, Microbial, 100 Mg/ml In Dmso, 0.2 Mum Filtered
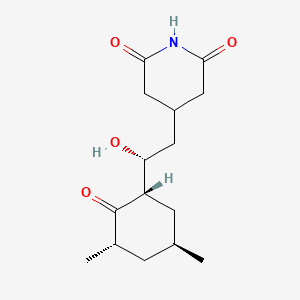
| Molecular Weight | 281.35 g/mol |
|---|---|
| Molecular Formula | C15H23NO4 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 281.16270821 g/mol |
| Monoisotopic Mass | 281.16270821 g/mol |
| Topological Polar Surface Area | 83.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 404 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Antifungal; Protein Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): ANTIBIOTIC, ANTIFUNGAL, TRICHOMONACIDAL ... ESPECIALLY EFFECTIVE AGAINST MALIGNANT LYMPHOMAS IN DOGS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 148
Cycloheximide ... was used in the treatment of cryptococcal infections (Cryptococcus neoformans) before the development of amphotericin B.
Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. 5(79) 489-513
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
STIMULATED TRANSPORT OF NUCLEAR RIBONUCLEOPROTEIN COMPLEXES TO CYTOPLASM AFTER ADMIN CYCLOHEXIMIDE TO RATS WAS STUDIED. ADMIN OF 2 MG/KG CYCLOHEXIMIDE CAUSED A DECR IN CONTENT OF TOTAL LIVER NUCLEAR RIBONUCLEOPROTEIN COMPLEX WITHIN 2 HR. THE OVERALL DECR WAS DUE TO AN INCREASED TRANSPORT INTO THE CYTOPLASM, NOT DECREASED SYNTHESIS. OTHER RESULTS SUGGEST THAT DURING INHIBITORY PHASE OF PROTEIN SYNTHESIS, GENE TRANSCRIPTION CONTINUES & GENE PRODUCT IS TRANSPORTED TO CYTOPLASM FOR TRANSLATION.
PMID:454372 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1186565 CHIH JJ ET AL; BIOCHEM J 178 (3): 643-9 (1979)
CYCLOHEXIMIDE (2-5 MG/KG BODY WT) CAUSED COMPLETE INHIBITION OF PROTEIN SYNTHESIS IN RAT LIVER WITHIN 30 MIN, & THE LABELING OF NUCLEAR PROTEINS WAS STRONGLY INHIBITED. UNDER THESE CONDITIONS, THE AMT OF NUCLEOLAR 45 S PRE-rRNA & ITS OROTATE-(14)C LABELING REMAINED UNAFFECTED FOR AT LEAST 4 HR, INDICATING THAT INITIALLY THE RATES OF SYNTHESIS & PROCESSING OF 45 S PRE-rRNA WERE NOT APPRECIABLY ALTERED. DRASTIC ALTERATIONS IN THE 45 S PRE-rRNA PROCESSING PATHWAYS OCCURRED AT THE EARLY STAGES OF CYCLOHEXIMIDE ACTION. THE CHANNELING OF NUCLEAR PRE-rRNA ALONG ALTERNATIVE PROCESSING PATHWAYS IS UNDER STRINGENT CONTROL BY THE CONTINUOUS SUPPLY OF CRITICAL PROTEINS.
PMID:456380 STOYANOVA BB, HADJIOLOV AA; EUR J BIOCHEM 96 (2): 349-56 (1979)
CYCLOHEXIMIDE IS A POTENT INHIBITOR OF PROTEIN SYNTHESIS IN FUNGI & ANIMALS. IT CAUSES AN INCREASE IN ADRENAL RNA, INCREASED PRODUCTION OF GLUCOCORTICOIDS ... & DECREASE IN PYRUVATE UTILIZATION IN ISOLATED ADIPOSE TISSUE.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-265