


1. Cyclogyl
1. 512-15-2
2. Cyclopentylate
3. Cyclogyl
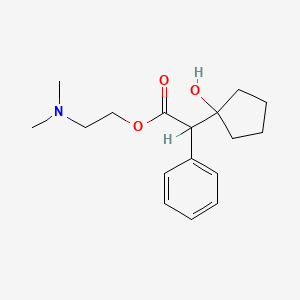
4. 2-(dimethylamino)ethyl 2-(1-hydroxycyclopentyl)-2-phenylacetate
5. Ciclopentolato
6. Cyclopentolatum
7. Cyclopentolatum [inn-latin]
8. Ciclopentolato [inn-spanish]
9. Mydrilate
10. Bell Pentolate
11. Cyclopentolate (inn)
12. 2-(dimethylamino)ethyl 1-hydroxy-alpha-phenylcyclopentaneacetate
13. I76f4shp7j
14. 1-hydroxy-alpha-phenylcyclopentaneacetic Acid 2-(dimethylamino)ethyl Ester
15. Benzeneacetic Acid, .alpha.-(1-hydroxycyclopentyl)-, 2-(dimethylamino)ethyl Ester
16. Chebi:4024
17. 2-(dimethylamino)ethyl (1-hydroxycyclopentyl)(phenyl)acetate
18. Alpha-(1-hydroxycyclopentyl)benzeneacetic Acid 2-(dimethylamino)ethyl Ester
19. Beta-dimethylaminoethyl (1-hydroxycyclopentyl)phenylacetate
20. Tsiklopentolat;cyclogyl;dl-cyclopentolate
21. Cyclopentoiate
22. Diopentolate
23. Beta-(dimethylamino)ethyl (1-hydroxycyclopentyl)phenylacetate
24. 2-phenyl-2-(1-hydroxycyclopentyl)ethanoic Acid Beta-(dimethylamino)ethyl Ester
25. Cyclopentolate [inn]
26. Cyclopentolate [inn:ban]
27. (+-)-cyclopentolate
28. Bell Pentolate (tn)
29. Einecs 208-136-4
30. Unii-i76f4shp7j
31. Cyclo-pentolatum
32. Benzeneacetic Acid, Alpha-(1-hydroxycyclopentyl)-, 2-(dimethylamino)ethyl Ester
33. Cyclogyl (salt/mix)
34. Mydrilate (salt/mix)
35. Spectrum_000856
36. (+/-)-cyclopentolate
37. 2-dimethylaminoethyl 2-(1-hydroxycyclopentyl)-2-phenylacetat
38. Prestwick0_001095
39. Prestwick1_001095
40. Prestwick2_001095
41. Prestwick3_001095
42. Spectrum2_001122
43. Spectrum3_000369
44. Spectrum4_000303
45. Spectrum5_000793
46. Cyclopentolate [mi]
47. Bspbio_001170
48. Bspbio_002097
49. Kbiogr_000886
50. Kbioss_001336
51. Cyclopentolate [vandf]
52. Divk1c_000610
53. Schembl132500
54. Spbio_000983
55. Spbio_003055
56. Bpbio1_001288
57. Gtpl7153
58. Cyclopentolate [who-dd]
59. Chembl1201338
60. Dtxsid3048528
61. Bdbm82375
62. Kbio1_000610
63. Kbio2_001336
64. Kbio2_003904
65. Kbio2_006472
66. Kbio3_001317
67. Ninds_000610
68. Bcp21334
69. Nsc_2905
70. Mfcd00599448
71. Stl430452
72. Akos015962137
73. Db00979
74. Idi1_000610
75. Cyclopentolatum; Cyclogyl; Cyclopentylate
76. Ncgc00018213-02
77. Ac-15991
78. As-16057
79. Cas_512-15-2
80. Sbi-0051324.p003
81. Ab00053445
82. Ft-0624265
83. C06932
84. D07759
85. H10434
86. Ab00053445_12
87. A913427
88. L000802
89. Q867332
90. Brd-a77291778-003-05-5
91. Brd-a77291778-003-15-4
92. .beta.-(dimethylamino)ethyl (1-hydroxycyclopentyl)phenylacetate
93. 2-(dimethylamino)ethyl (1-hydroxycyclopentyl)(phenyl)acetate #
94. 2-dimethylaminoethyl 1-hydroxy-.alpha.-phenylcyclopentaneacetate
95. (+)-alpha-(1-hydroxycyclopentyl)benzeneacetic Acid 2-(dimethylamino)ethyl Ester
96. (?)-alpha-(1-hydroxycyclopentyl)benzeneacetic Acid 2-(dimethylamino)ethyl Ester
97. 1-hydroxy-.alpha.-phenylcyclopentaneacetic Acid, 2-(dimethylamino)ethyl Ester
98. 2-(dimethylamino)ethyl (+/-)-1-hydroxy-.alpha.-phenylcyclopentaneacetate
99. Cyclopentaneacetic Acid, 1-hydroxy-.alpha.-phenyl-, 2-(dimethylamino)ethyl Ester
| Molecular Weight | 291.4 g/mol |
|---|---|
| Molecular Formula | C17H25NO3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 291.18344366 g/mol |
| Monoisotopic Mass | 291.18344366 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cyclogyl |
| PubMed Health | Atropine |
| Drug Classes | Anesthetic Adjunct, Cholinergic Antagonist, Gastrointestinal Agent, Nerve Gas Antidote, Urinary Antispasmodic |
| Drug Label | CYCLOGYL (cyclopentolate hydrochloride ophthalmic solution, USP) is an anticholinergic prepared as a sterile, borate buffered, solution for topical ocular use. It is supplied in three strengths. The active ingredient is represented by the structura... |
| Active Ingredient | Cyclopentolate hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5%; 1%; 2% |
| Market Status | Prescription |
| Company | Alcon; Alcon Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Pentolair |
| Active Ingredient | Cyclopentolate hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 3 of 4 | |
|---|---|
| Drug Name | Cyclogyl |
| PubMed Health | Atropine |
| Drug Classes | Anesthetic Adjunct, Cholinergic Antagonist, Gastrointestinal Agent, Nerve Gas Antidote, Urinary Antispasmodic |
| Drug Label | CYCLOGYL (cyclopentolate hydrochloride ophthalmic solution, USP) is an anticholinergic prepared as a sterile, borate buffered, solution for topical ocular use. It is supplied in three strengths. The active ingredient is represented by the structura... |
| Active Ingredient | Cyclopentolate hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.5%; 1%; 2% |
| Market Status | Prescription |
| Company | Alcon; Alcon Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Pentolair |
| Active Ingredient | Cyclopentolate hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
Used mainly to produce mydriasis and cycloplegia for diagnostic purposes.
Cyclopentolate is an anti-muscarinic in the same class as atropine and scopolamine. Cyclopentolate blocks the receptors in the muscles of the eye (muscarinic receptors). These receptors are involved controlling the pupil size and the shape of the lens. Cyclopentolate thus induces relaxation of the sphincter of the iris and the ciliary muscles. When applied topically to the eyes, it causes a rapid, intense cycloplegic and mydriatic effect that is maximal in 15 to 60 minutes; recovery usually occurs within 24 hours. The cycloplegic and mydriatic effects are slower in onset and longer in duration in patients who have dark pigmented irises.
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
S01FA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01F - Mydriatics and cycloplegics
S01FA - Anticholinergics
S01FA04 - Cyclopentolate
Absorption
Absorbed following ophthalmic administration.
By blocking muscarinic receptors, cyclopentolate produces dilatation of the pupil (mydriasis) and prevents the eye from accommodating for near vision (cycloplegia).