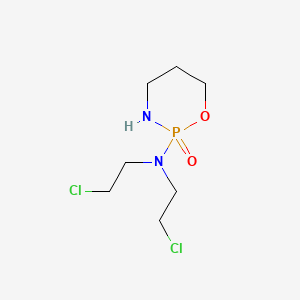

1. (+,-)-2-(bis(2-chloroethyl)amino)tetrahydro-2h-1,3,2-oxazaphosphorine 2-oxide Monohydrate
2. B 518
3. B-518
4. B518
5. Cyclophosphamide Anhydrous
6. Cyclophosphamide Monohydrate
7. Cyclophosphamide, (r)-isomer
8. Cyclophosphamide, (s)-isomer
9. Cyclophosphane
10. Cytophosphan
11. Cytophosphane
12. Cytoxan
13. Endoxan
14. Neosar
15. Nsc 26271
16. Nsc-26271
17. Nsc26271
18. Procytox
19. Sendoxan
1. 50-18-0
2. Cyclophosphamid
3. Cyclophosphane
4. Cytoxan
5. Cytophosphan
6. Procytox
7. Clafen
8. Endoxan
9. Cyclophosphan
10. Cyclostin
11. Neosar
12. Sendoxan
13. Cyclophosphamidum
14. Cytophosphane
15. Claphene
16. Endoxana
17. Endoxanal
18. Genoxal
19. Endoxan-asta
20. Endoxan R
21. Zyklophosphamid
22. Mitoxan
23. (rs)-cyclophosphamide
24. Cyclophosphoramide
25. Cyklofosfamid
26. Endoxane
27. Enduxan
28. Semdoxan
29. Senduxan
30. (+-)-cyclophosphamide
31. Cyclophosphamide Anhydrous
32. Cyclophosphanum
33. Rcra Waste Number U058
34. Asta B 518
35. 2h-1,3,2-oxazaphosphorin-2-amine, N,n-bis(2-chloroethyl)tetrahydro-, 2-oxide
36. Nsc 26271
37. Nsc-26271
38. Bis(2-chloroethyl)phosphoramide Cyclic Propanolamide Ester
39. B 518
40. Cb 4564
41. Nci-c04900
42. Sk 20501
43. Cyclofosphamide
44. Chebi:4027
45. N,n-bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide
46. (-)-cyclophosphamide
47. Cyclophosphamide (inn)
48. N,n-bis(2-chloroethyl)tetrahydro-2h-1,3,2-oxazaphosphorin-2-amine 2-oxide
49. Nsc26271
50. Anhydrous Cyclophosphamide
51. Cytoxan (tn)
52. 50-18-0 (anhydrous)
53. 2-[bis(2-chloroethylamino)]-tetrahydro-2h-1,3,2-oxazaphosphorine-2-oxide
54. Ciclophosphamide
55. 2-(bis(2-chloroethyl)amino)-2h-1,3,2-oxazaphosphorine 2-oxide
56. Asta
57. 6uxw23996m
58. N,n-bis(2-chloroethyl)-n',o-propylenephosphoric Acid Ester Diamide
59. N,n-di(2-chloroethyl)-n,o-propylene-phosphoric Acid Ester Diamide
60. 2h-1,3,2-oxazaphosphorin-2-amine, N,n-bis(2-chloroethyl)tetrahydro-,2-oxide
61. Phosphorodiamidic Acid, N,n-bis(2-chloroethyl)-n'-(3-hydroxypropyl)-, Intramol. Ester
62. Lyophilized Cytoxan
63. Cy
64. Ciclofosfamida
65. D,l-cyclophosphamide
66. Cyklofosfamid [czech]
67. N,n-bis(2-chloroethyl)-2-oxo-1,3,2$l^{5}-oxazaphosphinan-2-amine
68. Ciclophosphamide [inn]
69. 2-[bis(2-chloroethyl)amino]tetrahydro-2h-1,3,2-oxazaphosphorine 2-oxide
70. Zyklophosphamid [german]
71. Cyclophosphamide [inn]
72. Cyclophosphamide (anhydrous)
73. Asta B518
74. Ciclofosfamida [inn-spanish]
75. Cyclophosphamidum [inn-latin]
76. Occupation, Cyclophosphamide Exposure
77. Ccris 188
78. 4-hydroxy-cyclophosphan-mamophosphatide
79. Cyclophosphamide (anhydrous Form)
80. Cyclophosphamide (tn)
81. Hsdb 3047
82. Sr-01000075737
83. Ncgc00015209-05
84. Einecs 200-015-4
85. Rcra Waste No. U058
86. Brn 0011744
87. Cyclophosphamide [usp:inn]
88. 2-(bis(2-chloroethyl)amino)-2h-1,2-oxazaphosphorine 2-oxide
89. 2-[bis(2-chloroethyl)amino]-2h-1,2-oxazaphosphorine 2-oxide
90. Unii-6uxw23996m
91. 1-bis(2-chloroethyl)amino-1-oxo-2-aza-5-oxaphosphoridin
92. Ai3-26198
93. (bis(chloro-2-ethyl)amino)-2-tetrahydro-3,5,6-oxazaphosphorine-1,3,2-oxide-2 Hydrate
94. [bis(chloro-2-ethyl)amino]-2-tetrahydro-3,5,6-oxazaphosphorine-1,3,2-oxide-2 Hydrate
95. 173547-45-0
96. Cb-4564
97. Cyclophosphamide-[d4]
98. Spectrum_000858
99. Chembl88
100. Spectrum2_001146
101. Spectrum3_000370
102. Spectrum4_000304
103. Spectrum5_000795
104. Lopac-c-0768
105. N,n-bis(beta-chloroethyl)-n',o-propylenephosphoric Acid Ester Diamide
106. N,n-bis(beta-chloroethyl)-n',o-trimethylenephosphoric Acid Ester Diamide
107. Epitope Id:131782
108. 2-(bis(2-chloroethyl)amino)tetrahydro-2h-1,3,2-oxazophosphorine 2-oxide
109. C 0768
110. N,n-bis-(beta-chloraethyl)-n',o-propylen-phosphorsaeure-ester-diamid [german]
111. Schembl4346
112. (.+/-.)-cyclophosphamide
113. Lopac0_000238
114. Bspbio_002099
115. Kbiogr_000888
116. Kbioss_001338
117. Divk1c_000246
118. Spbio_001071
119. Cyclophosphamide [hsdb]
120. Gtpl7154
121. Schembl4345553
122. Dtxsid5020364
123. Schembl10262910
124. Cmsmoczeivjldb-uhfffaoysa-
125. Kbio1_000246
126. Kbio2_001338
127. Kbio2_003906
128. Kbio2_006474
129. Kbio3_001319
130. Cyclophospamide Monohydrate
131. Cyclophosphamide [who-dd]
132. Wln: T6mpotj Bo Bn2g2g
133. Cyclophosphamide, Anhydrous
134. Ninds_000246
135. 2-(bis(2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide
136. Cyclophosphamide Lyophilized
137. Hms2090a12
138. Hms3260p17
139. Hms3715j05
140. Pharmakon1600-01500213
141. Amy33449
142. Bcp02139
143. Tox21_500238
144. 2-chloro-5-methylbenzanilide
145. Bdbm50237604
146. N,n-bis(2-chloroethyl)-2-oxo-1,3,2lambda5-oxazaphosphinan-2-amine
147. N,n-bis-(beta-chloraethyl)-n',o-propylen-phosphorsaeure-ester-diamid
148. Nsc273033
149. Nsc273034
150. Nsc756711
151. Stk177249
152. 2h-1,3,2-oxazaphosphorine, 2-(bis(2-chloroethyl)amino)tetrahydro-, 2-oxide
153. Akos005410738
154. Cs-1425
155. Cyclophosphamide Anhydrous [mi]
156. Db00531
157. Lp00238
158. Nsc-273033
159. Nsc-273034
160. Sdccgsbi-0050226.p004
161. Idi1_000246
162. N,3,2-oxazaphosphorin-2-amine-2-oxide
163. N,o-propylen-phosphorsaeure-ester-diamid
164. Ncgc00015209-01
165. Ncgc00015209-02
166. Ncgc00015209-03
167. Ncgc00015209-04
168. Ncgc00015209-06
169. Ncgc00015209-07
170. Ncgc00015209-08
171. Ncgc00015209-09
172. Ncgc00015209-12
173. Ncgc00015209-28
174. Ncgc00091741-02
175. Ncgc00091741-03
176. Ncgc00260923-01
177. As-73255
178. Bp-25411
179. Hy-17420
180. Nci60_002097
181. N,o-propylenephosphoric Acid Ester Diamide
182. Sbi-0050226.p003
183. Db-082057
184. N,o-propylene-phosphoric Acid Ester Diamide
185. A2343
186. Eu-0100238
187. Ft-0624276
188. Ft-0665387
189. N,o-trimethylenephosphoric Acid Ester Diamide
190. En300-74526
191. C07888
192. D07760
193. Ab00053446-09
194. Ab00053446-11
195. Ab00053446-12
196. Ab00053446_13
197. Ab00053446_14
198. Ab00053446_15
199. 005c978
200. Q408524
201. W-60377
202. N,o-propylenephosphoric Acid Ester Amide Monohydrate
203. Sr-01000075737-1
204. Sr-01000075737-6
205. W-105248
206. Brd-a09722536-002-02-4
207. 2-(bis(2-chloroethyl)amino)-1,3,2-oxazaphosphinane2-oxide
208. 2-[bis(2-chloroethyl)amino]-1,3,2??-oxazaphosphinan-2-one
209. 2-[bis(2-chloroethyl)amino]-1,3,2$l^{5}-oxazaphosphinan-2-one
210. N,n-bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide #
211. 2-(di(2-chloroethyl)amino)-2-phospha(v)-tetrahydro-2h-1,3-oxazine-2-one
212. 2-[bis(2-chloroethyl)amino]tetrahydro-1,3,2-oxazaphosphorin-2-oxide
213. 2h-1,2-oxazaphosphorine, 2-[bis(2-chloroethyl)amino]tetrahydro-, 2-oxide
214. 2h-1,3,2-oxazaphosphorine, 2-(bis(2-chloroethyl)amino)tetrahydro-,2-oxide
215. N,n-bis(.beta.-chloroethyl)-n',o-trimethylenephosphoric Acid Ester Diamide
216. N,n-bis(2-chloroethyl)tetrahydro-2h-1,3,2-oxazaphosphorin-2-amine-2-oxide
217. N,n-bis-(.beta.-chloraethyl)-n',o-propylen-phosphorsaeure-ester-diamid
218. (+/-)-2-(bis(2-chloroethyl)amino)tetrahydro-2h-1,3,2-oxazaphosphorine 2-oxide
219. (+/-)-2-[bis(2-chloroethyl)amino]tetrahydro-2 H-1,3,2-oxazaphosphorine 2-oxide
220. (r)-2-[bis(2-chloroethyl)amino]tetrahydro-2h-1,3,2-oxazaphosphorin 2-oxide
221. (s)-2-[bis(2-chloroethyl)amino]tetrahydro-2h-1,3,2-oxazaphosphorine 2-oxide
222. 2h-1,2-oxazaphosphorin-2-amine, N,n-bis(2-chloroethyl)tetrahydro-, 2-oxide
223. 2h-1,2-oxazaphosphorin-2-amine, N,n-bis(2-chloroethyl)tetrahydro-, 2-oxide, (+)-
224. 2h-1,2-oxazaphosphorin-2-amine, N,n-bis(2-chloroethyl)tetrahydro-, 2-oxide, (-)-
225. 2h-1,2-oxazaphosphorine, 2-[bis(2-chloroethyl)amino]tetrahydro-, 2-oxide, Monohydrate
226. N,n-bis(2-chloroethyl)-2-oxo-1-oxa-3-aza-2$l^{5}-phosphacyclohexan-2-amine
227. Phosphorodiamidic Acid,n-bis(2-chloroethyl)-n'-(3-hydroxypropyl)-, Intramol. Ester
| Molecular Weight | 261.08 g/mol |
|---|---|
| Molecular Formula | C7H15Cl2N2O2P |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 260.0248201 g/mol |
| Monoisotopic Mass | 260.0248201 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 212 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cyclophosphamide |
| PubMed Health | Cyclophosphamide |
| Drug Classes | Alkylating Agent, Antineoplastic Agent, Antirheumatic, Cytotoxic, Nitrogen Mustard |
| Drug Label | Cyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. Cyclophosphamide is a white crystalline powder with the molecular formula C7H15Cl2N2O2P H2O and a molecular weight of 279.1. The chemical name for cy... |
| Active Ingredient | Cyclophosphamide |
| Dosage Form | Injectable; Tablet; Capsule |
| Route | Injection; Oral |
| Strength | 500mg/vial; 25mg; 50mg; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Roxane |
| 2 of 2 | |
|---|---|
| Drug Name | Cyclophosphamide |
| PubMed Health | Cyclophosphamide |
| Drug Classes | Alkylating Agent, Antineoplastic Agent, Antirheumatic, Cytotoxic, Nitrogen Mustard |
| Drug Label | Cyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. Cyclophosphamide is a white crystalline powder with the molecular formula C7H15Cl2N2O2P H2O and a molecular weight of 279.1. The chemical name for cy... |
| Active Ingredient | Cyclophosphamide |
| Dosage Form | Injectable; Tablet; Capsule |
| Route | Injection; Oral |
| Strength | 500mg/vial; 25mg; 50mg; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Roxane |
Alkylating Agents; Antineoplastic Agents, Alkylating; Antirheumatic Agents; Carcinogens; Immunosuppressive Agents; Mutagens; Teratogens
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): ... CYTOTOXIC AGENT FOR CARCINOMA, LEUKOSIS, ADENOMA, FIBROMA, & MIXED MAMMARY TUMORS OF DOGS ... MYCOTIC DERMATITIS ... OF SHEEP & TRYPANOSOMIASIS ... OF CATTLE ... ALSO ... CYTOTOXIC ... & IMMUNOSUPPRESSIVE IN RATS ... AGAINST EXPTL ALLERGIC ENCEPHALOMYELITIS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 148
The clinical spectrum of activity for cyclophosphamide is very broad. It is an essential component of many effective drug combinations for non-Hodgkin's lymphomas. Complete remissions & presumed cures have been reported when cyclophosphamide was given as a single agent for Burkitt's lymphoma. It is frequently used in combination with methotrexate (or doxorubicin) & fluorouracil as adjuvant therapy after surgery for carcinoma of the breast. Notable advantages of this drug are the availability of the oral route of admin & the possibility of giving fractionated doses over prolonged periods. For these reasons it possesses a versatility of action that allows an intermediate range of use, between that of the highly reactive iv mechlorethamine & that of oral chlorambucil. Beneficial results have been obtained in multiple myeloma; chronic lymphocytic leukemia; carcinomas of the lung, breast, cervix, & ovary; & neuroblastoma, retinoblastoma, & other neoplasms of childhood. Because of its potent immunosuppressive properties, cyclophosphamide has received considerable attention for the control of organ refection after transplantation & in nonneoplastic disorders associated with altered immune reactivity, including Wegener's granulomatosis, rheumatoid arthritis, & the nephrotic syndrome in children.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1395
Furosemide may improve, renal blood flow, decrease resorption of sodium and chloride, and increase free water excretion. The initial dose of furosemide is 2 mg/kg, IV. This dosage can be doubled or tripled if urine output does not increase within 1 hr. However, if there is no response to 6 mg/kg, another approach should be tried. If effective, furosemide can be given parenterally at 2 mg/kg, tid., to maintain a diuresis.
Aiello, S.E. (ed). The Merck Veterinary Manual. 8th ed. Merck & Co., Inc., National Publishing Inc., Philadelphia, PA. 1998., p. 1732
For more Therapeutic Uses (Complete) data for CYCLOPHOSPHAMIDE (30 total), please visit the HSDB record page.
Appropriate caution is advised when the drug is considered for use in ... /nonneoplastic/ conditions, not only because of its acute toxic effects but also because of its high potential for inducing sterility, teratogenic effects, & leukemia. ... Administration of the drug should be interrupted at the first indication of dysuria or hematuria. The syndrome of inappropriate secretion of antidiuretic hormone (ADH) has been observed in patients receiving cyclophosphamide, usually at doses higher than 50 mg/kg. It is important to be aware of the possibility of water intoxication, since these patients are usually vigorously hydrated.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1395
POTENTIAL ADVERSE EFFECTS ON FETUS: Various fetal malformations, especially skeletal defects and dysmorphic features, but other chemotherapeutic agents given concurrently. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: Transient neutropenia from cyclophosphamide with prednisone and vincristine. Potential mutagenicity, carcinogenicity, adverse effects on fetus. FDA Category: D (D = There is evidence of human fetal risk, but the potential benefits from use in pregnant women may be acceptable despite the potential risks (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective.)) /from Table II/
PMID:2195076 Stockton DL and Paller AS; J Am Acad Dermatol 23 (1):87-103 (1990)
Drugs that are Contraindicated during Breast-Feeding: Cyclophosphamide: Possible immune suppression; unknown effect on growth or association with carcinogenesis; neutropenia. /from Table 1./
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 138 (1994)
THE DRUG IS MOST TOXIC TO THE HUMAN FETUS DURING 1ST 3 MO & CONGENITAL ABNORMALITIES HAVE BEEN DETECTED AFTER IV INJECTION OF LARGE DOSES TO PREGNANT WOMEN DURING THIS PERIOD OF PREGNANCY. /MONOHYDRATE/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 146 (1975)
For more Drug Warnings (Complete) data for CYCLOPHOSPHAMIDE (27 total), please visit the HSDB record page.
Cyclophosphamide is indicated for the treatment of malignant lymphomas, multiple myeloma, leukemias, mycosis fungoides (advanced disease), neuroblastoma (disseminated disease), adenocarcinoma of the ovary, retinoblastoma, and carcinoma of the breast. It is also indicated for the treatment of biopsy-proven minimal change nephrotic syndrome in pediatric patients.
Treatment of malignant diseases
Treatment of all malignant neoplasms
Cyclophosphamide is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
Myeloablative Agonists
Agents that destroy bone marrow activity. They are used to prepare patients for BONE MARROW TRANSPLANTATION or STEM CELL TRANSPLANTATION. (See all compounds classified as Myeloablative Agonists.)
Antirheumatic Agents
Drugs that are used to treat RHEUMATOID ARTHRITIS. (See all compounds classified as Antirheumatic Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AA - Nitrogen mustard analogues
L01AA01 - Cyclophosphamide
Absorption
After oral administration, peak concentrations occur at one hour.
Route of Elimination
Cyclophosphamide is eliminated primarily in the form of metabolites. 10-20% is excreted unchanged in the urine and 4% is excreted in the bile following IV administration.
Volume of Distribution
30-50 L
Clearance
Total body clearance = 63 7.6 L/kg.
Cyclophosphamide is well absorbed orally.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1395
PLACENTAL TRANSFER OF (14)CARBON-CYCLOPHOSPHAMIDE HAS BEEN DEMONSTRATED IN MICE; AND A POSITIVE CORRELATION BETWEEN THE ALKYLATION OF EMBRYONIC DNA AND PRODUCTION OF CONGENITAL ABNORMALITIES IN MICE HAS BEEN REPORTED. A SIMILAR CORRELATION HAS BEEN FOUND FOR NUCLEAR-DNA-DEPENDENT RNA POLYMERASES IN RAT EMBRYOS. IN MOST SPECIES, CYCLOSPHSPHAMIDE IS RAPIDLY ABSORBED, METABOLIZED AND EXCRETED. IN RATS, THE SPECIFIC ACTIVITY IN TISSUES IS HIGHEST WITHIN 20-30 MIN FOLLOWING IP INJECTION; UP TO 75% OF THE RADIOACTIVITY IS EXCRETED WITHIN 5-8 HR. /MONOHYDRATE/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 176 (1981)
AFTER ITS IV INJECTION, THE DRUG IS RAPIDLY ABSORBED FROM THE BLOOD. IN PATIENTS RECEIVING 6.7-80 MG/KG BODY WT PER DAY OF RING LABELLED CYCLOPHOSPHAMIDE, RADIOACTIVITY WAS DISTRIBUTED RAPIDLY TO ALL TISSUES: ITS HALF LIFE IN THE PLASMA WAS 6.5 HOURS. NO RADIOACTIVITY WAS FOUND IN THE EXPIRED AIR OR FECES. RECOVERY OF RADIOACTIVITY IN URINE HAS BEEN REPORTED TO BE BETWEEN 50-68%, MAINLY IN THE FORM OF CARBOXYPHOSPHAMIDE AND PHOSPHORAMIDE MUSTARD; 10-40% OF THE DRUG WAS EXCRETED UNCHANGED; AND 56% OF THE REACTIVE METABOLITES WERE BOUND TO PLASMA PROTEINS. /MONOHYDRATE/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 181 (1981)
In a cross sectional study, the urine of 20 hospital workers occupationally exposed to cyclophosphamide and 21 unexposed controls was monitored for excretion of cyclophosphamide. During the week in which samples were collected, most of the workers handled cyclophosphamide fewer than 5 times and the amount handled each time ranged from 100-1000 mg (mean + or - 350 mg). All workers claimed to have taken regular safety precautions; ie, at least wearing gloves during handling. The drug was identified in 5 cases (range: 0.7-2.5 ug cyclophosphamide excreted/24 hr urine). A clear relationship between cyclophosphamide handling and urinary detection was shown. 4 of 5 persons with detectable urinary cyclophosphamide had handled cyclophosphamide 10 times or more during the week.
PMID:3744569 Evelo CTA et al; Int Arch Occup Environ Health 58: 151-5 (1986)
For more Absorption, Distribution and Excretion (Complete) data for CYCLOPHOSPHAMIDE (7 total), please visit the HSDB record page.
Metabolism and activation occurs at the liver. 75% of the drug is activated by cytochrome P450 isoforms, CYP2A6, 2B6, 3A4, 3A5, 2C9, 2C18, and 2C19. The CYP2B6 isoform is the enzyme with the highest 4-hydroxylase activity. Cyclophosphamide undergoes activation to eventually form active metabolites, phosphoramide mustard and acrolein. Cyclophosphamide appears to induce its own metabolism which results in an overall increase in clearance, increased formation of 4-hydroxyl metabolites, and shortened t1/2 values following repeated administration.
... /Cyclophosphamide/ is activated by the hepatic cytochrome P450 system. Cyclophosphamide is first converted to 4-hydroxycyclophosphamide, which is in a steady state with the acyclic tautomer aldophosphamide. In vitro studies with human liver microsones & cloned P450 isoenzymes have shown that cyclophosphamide is activated by the CYP2B group of P450 isoenzymes... . 4-hydroxycyclophosphamide may be oxidized further by aldehyde oxidase either in liver or in tumor tissue & perhaps by other enzymes, yielding the metabolites carboxyphosphamide & 4-ketocyclophsphamide, neither of which possesses significant biological activity. It appears that hepatic damage is minimized by theses secondary reactions, whereas significantl amoutns of the active metabolies, such as 4-hydroxycyclophosphamide & its tautomer, aldophosphamed, are transported to the target sites by the circulatory system. In tumor cells, the aldophosphamide cleaves spontaneously, generating stoichiometric amounts of phosphoramide mustard & acrolein. The former is believed to be responsible for antitumor effects. The latter cmpd may be responsible for the hemorrhagic cystitis seen during therapy with cyclophosphamide. Cystitis can be reduced in intensity or prevented by the pareneteral admin of mesna, a sulfhydryl cmpd that reacts readily with acrolein in the acid environment of the urinary tract. ... Urinary & fecal recovery of unchanged cyclophosphamide is minimal after iv admin. Maximal concns in plasma are achieved 1 hr after oral admin, & the half-life in plasma is about 7 hr.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1395
SHEEP WERE ORALLY DOSED WITH CYCLOPHOSPHAMIDE. IN COLLECTED URINE, 2 METABOLITES WERE OBSERVED AND CHARACTERIZED AS O-(2-CARBOXYETHYL)-N,N-BIS (2-CHLOROETHYL)PHOSPHORODIAMIDATE & 2-(BIS (2-CHLOROETHYL)AMINO)TETRAHYDRO-2H-1,3,2-OXAZOPHOSPHORINE 2,4-DIOXIDE (4-KETOCYCLOPHOSPHAMIDE).
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 119
A REACTIVE METABOLITE, N,N-BIS-(2-CHLOROETHYL)PHOSPHORODIAMIDIC ACID, WHICH POSSESSES POTENT ALKYLATING & CYTOTOXIC PROPERTIES, HAS RECENTLY BEEN ISOLATED FROM THE OXYGENATION PRODUCTS OF CYCLOPHOSPHAMIDE AND MOUSE LIVER MICROCHROMOSOMES.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 473
Cyclophosphamide is well absorbed orally, and peak plasma levels appear about one hour after oral use. It is also administered intravenously. This drug is metabolized in the liver to the cytotoxic metabolite, 4-hydroxycyclophosphamide, which is in equilibrium with the acyclic tautomer, aldophosphamide. Although the major fraction of these metabolites is oxidized further to inactive products, some aldophosphamide is converted to phophoramidemustard, which alkylates DNA, and to acrolein.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 2012
For more Metabolism/Metabolites (Complete) data for CYCLOPHOSPHAMIDE (8 total), please visit the HSDB record page.
3-12 hours
Maximal concns in plasma are achieved 1 hr after oral admin, & the half-life in plasma is about 7 hr.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1395
Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases, preventing DNA synthesis and RNA transcription from the affected DNA, 2) DNA damage via the formation of cross-links (bonds between atoms in the DNA) which prevents DNA from being separated for synthesis or transcription, and 3) the induction of mispairing of the nucleotides leading to mutations.
The chemotherapeutic alkylating agents have in common the property of becoming strong electrophiles through the formation of carbonium ion intermediates or of transition complexes with the target molecules. These reactions result in the formation of covalent linkages by alkylation of various nucleophilic moieties such as phosphate, amino, sulfhydryl, hydroxyl, carboxyl, & imidazole groups. The chemotherapeutic & cytotoxic effects are directly related to the alkylation of DNA. The 7 nitrogen atom of guanine is particularly susceptible to the formation of a covalent bond with bifunctional alkylating agents & may well represent the key target that determines their biological effects. It must be appreciated, however, that other atoms in the purine & pyrimidine bases of DNA- particularly, the 1 & 3 nitrogens of adenine, the 3 nitrogen of cytosine, & the 6 oxygen of guanine- also may be alkylated, as will be the phosphate atoms of the DNA chains & amino & sulfhydryl groups of proteins. /Alkylating agents/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1389
Cyclophosphamide can be used to cause immunologically mediated regression of the immunogenic, cyclophosphamide-resistant L5178Y lymphoma in syngeneic and semisyngeneic mice (B6D2F1 (C57BL/6 x DBA/2) females). In order to cause tumor regression it was necessary to give cyclophosphamide (125-200 mg/kg of body wt, iv shortly before or shortly after tumor implantation. Regardless of whether cyclophosphamide was given before or after tumor implantation, tumor regression was associated with the presence in the spleen of an incr number of Lyt-2+ T-cells capable of passively transferring immunity to tumor bearing recipients. This augmented level of immunity was sustained throughout the period of tumor regression. In contrast, a lower level of concomitant immunity generated by control tumor bearers decayed after day 12 of tumor growth. Because the therapeutic effect of cyclophosphamide could be inhibited by passive transfer of L3T4+ T-cells from normal donor mice it is apparent that the therapeutic effect of cyclophosphamide is based on its ability to preferentially destroy L3T4+ suppressor T-cells. These putative precursor suppressor T-cells were regenerated 4 days after being destroyed by cyclophosphamide.
PMID:2522344 Awwad M, North RJ; Cancer Res 49 (7): 1649-54 (1989)
These studies enable the pattern of emesis and nausea for 3 days following high-dose cyclophosphamide to be described and give some insight into the mechanisms of emesis which may be operating. Nausea and vomiting induced by cyclophosphamide-based chemotherapy has long latency of onset (8-13 hr) and continues for at least 3 days. These findings are of particular importance as many of these patients receive chemotherapy as outpatients and emphasize the need for appropriate anti-emetic prophylaxis for patients at home. Ondansetron was extremely effective over this time in the control of emesis and nausea. These results suggest that high-dose cyclophosphamide-induced emesis over days 1-3 is largely mediated via 5-hydroxytryptamine (5-HT) and 5-HT3 receptors.
Beck TM; Anticancer Dugs 6 (2): 237-42 (1995)
The most likely mechanism by which cyclophosphamide augments immune responses relates to preferential elimination of suppressor and relative sparing of effector and helper cells. Thus, precursors and mature murine suppressor cells are very sensitive to cyclophosphamide whereas the mature effector cells are relatively insensitive ... . Cyclophosphamide induced immunological regression of murine leukemia is reversed by the infusion of normal spleen cells as a source of precursors of suppressor cells ... . Memory and helper T cells are relatively resistant to the cytotoxic effect of cyclophosphamide ... . NK activity against YAC lymphoma targets by non T and non B cells is depressed by cyclophosphamide ... .
Foye, W.O. (ed.). Cancer Chemotherapeutic Agents. ACS Professional Reference Book. Washington, D.C.: American Chemical Society, 1995., p. 469