


1. 114, Tmc
2. Darunavir
3. Ethanolate, Darunavir
4. Prezista
5. Tmc 114
6. Tmc-114
7. Tmc114
8. Uic 94017
9. Uic-94017
10. Uic94017
1. 635728-49-3
2. Darunavir (ethanolate)
3. Prezista
4. Rezolsta
5. Unii-33o78xf0bw
6. Tmc114 Ethanolate
7. Darunavir Monoethanolate
8. Darunavir (as Ethanolate)
9. 33o78xf0bw
10. Darunavir Ethanolate (prezista)
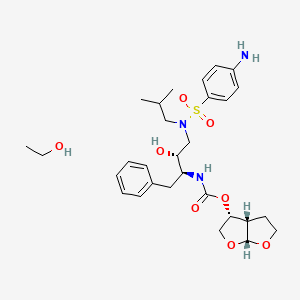
11. [(3as,4r,6ar)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2s,3r)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate;ethanol
12. Darunavir Ethanolate (jan)
13. Darunavir Ethanolate [jan]
14. Carbamic Acid, ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3r,3as,6ar)-hexahydrofuro(2,3-b)furan-3-yl Ester, Compd. With Ethanol (1:1)
15. Uic 94017
16. [14c]-darunavir Ethanolate
17. Prezista (tn)
18. Darunavir?ethanolate
19. Darunavir Ethanolate- Bio-x
20. Schembl562454
21. Chembl1201127
22. Dtxsid70979794
23. Symtuza Component Darunavir
24. Amy39006
25. Darunavir Ethanolate [vandf]
26. Darunavir Ethanolate [mart.]
27. Darunavir Monoethanolate [mi]
28. Mfcd18251642
29. Darunavir Component Of Symtuza
30. Darunavir Ethanolate [who-dd]
31. Akos025149225
32. Bcp9000588
33. Ccg-270170
34. Cs-0750
35. Ac-26777
36. As-35215
37. Bd164352
38. Darunavir Ethanolate [orange Book]
39. Hy-17041
40. S1620
41. Sw219052-1
42. D06478
43. Prezcobix Component Darunavir Ethanolate
44. A847955
45. Darunavir Ethanolate Component Of Prezcobix
46. Q27256287
47. ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)- Carbamic Acid (3r,3as,6ar)-hexahydrofuro(2,3-b)furan-3-yl Ester Monoethanolate
48. (3r,3as,6ar)-hexahydrofuro(2,3-b)furan-3-yl ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(isobutyl)amino)-1-benzyl-2-hydroxypropyl)carbamate - Ethanol (1:1)
49. (3r,3as,6ar)-hexahydrofuro[2,3-b]furan-3-yl ((2s,3r)-4-(4-amino-n-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-yl)carbamate Ethanolate
50. (3r,3as,6ar)-hexahydrofuro[2,3-b]furan-3-yl((2s,3r)-4-((4-amino-n-isobutylphenyl)sulfonamido)-3-hydroxy-1-phenylbutan-2-yl)carbamatecompoundwithethanol(1:1)
51. [(3as,4r,6ar)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2s,3r)-4-[(4-aminophenyl) Sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate; Ethanol; Tmc114
52. 635728-39-1
53. Hexahydrofuro[2,3-b]furan-3-yl Hydrogen {4-[(4-aminobenzene-1-sulfonyl)(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl}carbonimidate--ethanol (1/1)
54. N-[(1s,2r)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid (3r,3as,6ar)-hexahydrofuro[2,3-b]furan-3-yl Ester Compd. With Ethanol
| Molecular Weight | 593.7 g/mol |
|---|---|
| Molecular Formula | C29H43N3O8S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 593.27708651 g/mol |
| Monoisotopic Mass | 593.27708651 g/mol |
| Topological Polar Surface Area | 169 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 856 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Darunavir ethanolate |
| Drug Label | PREZISTA (darunavir) is an inhibitor of the human immunodeficiency virus (HIV) protease.PREZISTA (darunavir), in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-... |
| Active Ingredient | Darunavir ethanolate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg; 150mg; 600mg; 400mg |
| Market Status | Tentative Approval |
| Company | Teva Pharms Usa; Hetero Labs Unit Iii |
| 2 of 2 | |
|---|---|
| Drug Name | Darunavir ethanolate |
| Drug Label | PREZISTA (darunavir) is an inhibitor of the human immunodeficiency virus (HIV) protease.PREZISTA (darunavir), in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-... |
| Active Ingredient | Darunavir ethanolate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg; 150mg; 600mg; 400mg |
| Market Status | Tentative Approval |
| Company | Teva Pharms Usa; Hetero Labs Unit Iii |
Rezolsta, is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus 1 (HIV 1) infection in adults aged 18 years or older.
Genotypic testing should guide the use of Rezolsta.
PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adult and paediatric patients from the age of 3 years and at least 15 kg body weight.
PREZISTA, co administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adults and adolescents (aged 12 years and older, weighing at least 40 kg).
In deciding to initiate treatment with PREZISTA co administered with cobicistat or low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of PREZISTA.
PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV 1) infection.
PREZISTA 75 mg, 150 mg, and 600 mg tablets may be used to provide suitable dose regimens:
- For the treatment of HIV 1 infection in antiretroviral treatment (ART) experienced adult patients, including those that have been highly pre treated.
- For the treatment of HIV 1 infection in paediatric patients from the age of 3 years and at least 15 kg body weight.
In deciding to initiate treatment with PREZISTA co administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of PREZISTA.
PREZISTA, co administered with low dose ritonavir is indicated in combination with other antiretroviral medicinal products for the treatment of patients with human immunodeficiency virus (HIV 1) infection.
PREZISTA, co administered with cobicistat is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection in adults and adolescents (aged 12 years and older, weighing at least 40 kg).
PREZISTA 400 mg and 800 mg tablets may be used to provide suitable dose regimens for the treatment of HIV 1 infection in adult and paediatric patients from the age of 3 years and at least 40 kg body weight who are:
- antiretroviral therapy (ART) nave.
- ART experienced with no darunavir resistance associated mutations (DRV RAMs) and who have plasma HIV 1 RNA < 100,000 copies/ml and CD4+ cell count 100 cells x 106/L. In deciding to initiate treatment with PREZISTA in such ART experienced patients, genotypic testing should guide the use of PREZISTA.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05
J05AE10