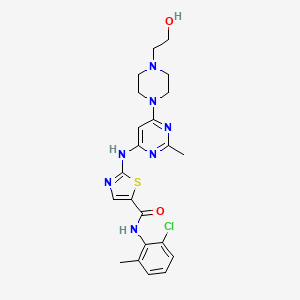

1. (18f)-n-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
2. 354825, Bms
3. Bms 354825
4. Bms-354825
5. Bms354825
6. N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
7. Sprycel
1. 302962-49-8
2. Sprycel
3. Bms-354825
4. Dasatinib Anhydrous
5. Bms 354825
6. Bms354825
7. Dasatinib (bms-354825)
8. Dasatinib (anhydrous)
9. N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide
10. N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
11. N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxamide
12. N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide
13. Anh. Dasatinib
14. Anhydrous Dasatinib
15. Dasatinib (anh.)
16. Chembl1421
17. X78ug0a0rn
18. Chebi:49375
19. Dasatinib D8
20. Nsc732517
21. Nsc-732517
22. Nsc-759877
23. Ncgc00181129-01
24. Dsstox_cid_20979
25. Dsstox_rid_79608
26. Dsstox_gsid_40979
27. 5-thiazolecarboxamide, N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)-1-piperazinyl)-2-methyl-4-pyrimidinyl)amino)-
28. Dasatinibum
29. Bms-354825 Hydrate
30. 5-thiazolecarboxamide, N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-
31. Smr002529551
32. Cas-302962-49-8
33. 302962-49-8 Pound Not863127-77-9
34. Dasatinib [usan:inn]
35. Unii-x78ug0a0rn
36. Bms Dasatinib
37. 1n1
38. Kinome_3650
39. Dasatinib (jan/inn)
40. Dasatinib [inn]
41. Dasatinib [mi]
42. N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide
43. Sprycel (bristol Meyers)
44. Dasatinib [who-dd]
45. Schembl8226
46. Dasatinib,bms-354825
47. Mls003915609
48. Mls004774145
49. Mls006010904
50. Dasatinib - Bms-354825
51. Gtpl5678
52. Dtxsid4040979
53. Bdbm13216
54. Cid_3062316
55. Ex-a401
56. 5-thiazolecarboxamide, Monohydrate
57. Bcpp000263
58. Hms2043n05
59. Hms3244a05
60. Hms3244a06
61. Hms3244b05
62. Hms3265c19
63. Hms3265c20
64. Hms3265d19
65. Hms3265d20
66. Hms3654k05
67. Hms3744c11
68. Pharmakon1600-01502275
69. Bcp01797
70. Zinc3986735
71. Tox21_112736
72. Mfcd11046566
73. Nsc759877
74. Nsc800087
75. S1021
76. Akos015902363
77. Tox21_112736_1
78. Bcp9000589
79. Bms 345825
80. Ccg-264779
81. Cs-0100
82. Db01254
83. Gs-6548
84. Nsc-800087
85. Sb17284
86. Ncgc00181129-02
87. Ncgc00181129-03
88. Ncgc00181129-05
89. Ncgc00181129-06
90. Ncgc00181129-07
91. Ncgc00181129-12
92. Ncgc00181129-14
93. Ncgc00181129-22
94. Ncgc00481571-01
95. 2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)-n-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
96. Ac-22749
97. Bcb03_000715
98. Bms 35482513
99. Hy-10181
100. N-(2-chloro-6-methyl-phenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methyl-pyrimidin-4-yl]amino]thiazole-5-carboxamide
101. Am20080877
102. D5949
103. Ft-0650671
104. Sw208076-5
105. Ec-000.2122
106. D-3307
107. D03658
108. Ab01273956-01
109. Ab01273956-02
110. Ab01273956_03
111. Ar-270/43507994
112. Q419940
113. Sr-00000000554
114. Q-101345
115. Sr-00000000554-5
116. Brd-k49328571-001-05-1
117. Brd-k49328571-001-07-7
118. Bms-354825;bms354825;bms 354825
119. Z2786158251
120. N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl}amino)-1,3-thiazole-5-carboxamide
121. N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide
| Molecular Weight | 488.0 g/mol |
|---|---|
| Molecular Formula | C22H26ClN7O2S |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 487.1557220 g/mol |
| Monoisotopic Mass | 487.1557220 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Sprycel |
| PubMed Health | Dasatinib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Drug Label | SPRYCEL (dasatinib) is a kinase inhibitor. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C22H26ClN7O2... |
| Active Ingredient | Dasatinib |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 140mg; 100mg; 50mg; 80mg; 70mg; 20mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 2 | |
|---|---|
| Drug Name | Sprycel |
| PubMed Health | Dasatinib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Drug Label | SPRYCEL (dasatinib) is a kinase inhibitor. The chemical name for dasatinib is N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate. The molecular formula is C22H26ClN7O2... |
| Active Ingredient | Dasatinib |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 140mg; 100mg; 50mg; 80mg; 70mg; 20mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
For the treatment of adults with chronic, accelerated, or myeloid or lymphoid blast phase chronic myeloid leukemia with resistance or intolerance to prior therapy. Also indicated for the treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy.
FDA Label
Sprycel is indicated for the treatment of paediatric patients with:
newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukaemia in chronic phase (Ph+ CML CP) or Ph+ CML CP resistant or intolerant to prior therapy including imatinib. newly diagnosed Ph+ acute lymphoblastic leukaemia (ALL) in combination with chemotherapy.
Sprycel is indicated for the treatment of adult patients with:
- newly diagnosed Philadelphia-chromosome-positive (Ph+) chronic myelogenous leukaemia (CML) in the chronic phase;
- chronic, accelerated or blast phase CML with resistance or intolerance to prior therapy including imatinib mesilate;
- Ph+ acute lymphoblastic leukaemia (ALL) and lymphoid blast CML with resistance or intolerance to prior therapy.
Sprycel is indicated for the treatment of paediatric patients with:
- newly diagnosed Ph+ CML in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib.
Treatment of Philadelphia-chromosome (BCR-ABL translocation)-positive acute lymphoblastic leukaemia, Treatment of Philadelphia-chromosome (BCR-ABL translocation)-positive chronic myeloid leukaemia
Dasatinib Accord is indicated for the treatment of adult patients with:
Ph+ acute lymphoblastic leukaemia (ALL) with resistance or intolerance to prior therapy.
Dasatinib Accord is indicated for the treatment of paediatric patients with:
newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma is indicated for the treatment of adult patients with:
newly diagnosed Philadelphia chromosome positive (Ph+) chronic myelogenous leukaemia (CML) in the chronic phase.
chronic, accelerated or blast phase CML with resistance or intolerance to prior therapy including imatinib.
Ph+ acute lymphoblastic leukaemia (ALL) and lymphoid blast CML with resistance or intolerance to prior therapy.
Dasatinib Accordpharma is indicated for the treatment of paediatric patients with:
newly diagnosed Ph+ CML in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib.
newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib is an oral dual BCR/ABL and Src family tyrosine kinase inhibitor
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L01EA02
L01EA02
L01EA02
L01XE06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EA - Bcr-abl tyrosine kinase inhibitors
L01EA02 - Dasatinib
Route of Elimination
Dasatinib is extensively metabolized in humans, primarily by the cytochrome P450 enzyme 3A4. Elimination is primarily via the feces.
Volume of Distribution
2505 L
Dasatinib is extensively metabolized in humans, primarily by the cytochrome P450 enzyme 3A4
The overall mean terminal half-life of dasatinib is 3-5 hours.
Dasatinib, at nanomolar concentrations, inhibits the following kinases: BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ. Based on modeling studies, dasatinib is predicted to bind to multiple conformations of the ABL kinase. In vitro, dasatinib was active in leukemic cell lines representing variants of imatinib mesylate sensitive and resistant disease. Dasatinib inhibited the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. Under the conditions of the assays, dasatinib was able to overcome imatinib resistance resulting from BCR-ABL kinase domain mutations, activation of alternate signaling pathways involving the SRC family kinases (LYN, HCK), and multi-drug resistance gene overexpression.