


1. Cv 3317
2. Cv-3317
3. Delapril
4. Derapril
5. N-(2,3-dihydro-1h-inden-2-yl)-n-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)alanyl)glycine
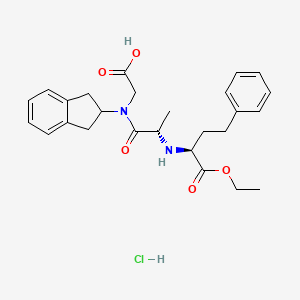
1. Delapril Hcl
2. 83435-67-0
3. Adecut
4. Rev 6000a
5. Cv 3317
6. Rev-6000a
7. 2smm3m5zmh
8. Cv-3317
9. Ethyl (s)-2-(((s)-1-((carboxymethyl)-2-indanylcarbamoyl)ethyl)amino)-4-phenylbutyrate, Monohydrochloride
10. Delapril (hydrochloride)
11. 2-[2,3-dihydro-1h-inden-2-yl-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]amino]acetic Acid;hydrochloride
12. Glycine, N-(2,3-dihydro-1h-inden-2-yl)-n-(n-(1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl)-, Monohydrochloride, (s)-
13. Indalapril
14. 2-((s)-n-(2,3-dihydro-1h-inden-2-yl)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanamido)acetic Acid Hydrochloride
15. 2-[(2s)-n-(2,3-dihydro-1h-inden-2-yl)-2-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanamido]acetic Acid Hydrochloride
16. Ccris 1925
17. Delapril Hydrochloride [usan:jan]
18. Ncgc00181755-01
19. Unii-2smm3m5zmh
20. Cupressin
21. Delaket
22. Adecut (tn)
23. Delapril?hydrochloride
24. Alindapril Hydrochloride
25. Dsstox_cid_28523
26. Dsstox_rid_82795
27. N-(n-((s)-1-ethoxycarbonyl-3-phenylpropyl)-l-alanyl)-n-(indan-2-yl)glycine Hydrochloride
28. Dsstox_gsid_48597
29. Glycine, N-((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)-l-alanyl-n-(2,3-dihydro-1h-inden-2-yl)-, Monohydrochloride
30. Schembl120907
31. Chembl2106126
32. Dtxsid6048597
33. Chebi:31462
34. Delapril Hydrochloride [mi]
35. Delapril Hydrochloride (jan/usan)
36. Delapril Hydrochloride [jan]
37. Tox21_112927
38. Delapril Hydrochloride [usan]
39. Mfcd00884619
40. S5728
41. Akos015915580
42. Delapril Hydrochloride [mart.]
43. Ccg-269602
44. Delapril Hydrochloride [who-dd]
45. As-15996
46. Cas-83435-67-0
47. Hy-107337
48. Cs-0028174
49. D4082
50. D01667
51. Q27255552
52. N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-(s)-alanyl-n-(2-indanyl)glycine Hydrochloride
53. N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-l-alanyl-n-(indan-2-yl)glycine Hydrochloride
54. 2-((s)-n-(2,3-dihydro-1h-inden-2-yl)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanamido)acetic Acid Hydrochloride
55. Glycine,n-[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]-l-alanyl-n-(2,3-dihydro-1h-inden-2-yl)-, Monohydrochloride
56. N-[(s)-1-ethoxycarbonyl-3-phenylpropyl]-l-alanyl-n-(2,3-dihydroinden-2-yl)glycine Hydrochloride
| Molecular Weight | 489.0 g/mol |
|---|---|
| Molecular Formula | C26H33ClN2O5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 12 |
| Exact Mass | 488.2077999 g/mol |
| Monoisotopic Mass | 488.2077999 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 649 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)