

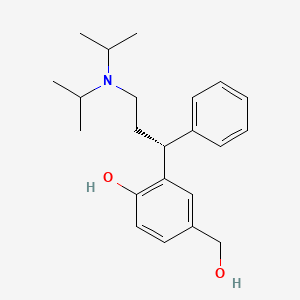

1. (r)-n,n-diisopropyl-3-(2-hydroxy-5-hydroxymethylphenyl)-3-phenylpropanamine
2. 5-hydroxymethyl Tolterodine
3. Pnu 200577
4. Pnu-200577
1. 207679-81-0
2. (r)-5-hydroxymethyl Tolterodine
3. 5-hydroxymethyl Tolterodine
4. (r)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenol
5. Pnu-200577
6. 5-hydroxymethyltolterodine
7. Desfesoterodine [inn]
8. 5-hmt
9. Pnu 200577
10. 3-[(1r)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxybenzenemethanol
11. 5-hydroxymethyl Tolterodine (pnu 200577, 5-hmt, 5-hm)
12. Spm 7605
13. Yu871o78gr
14. (+)-n,n-diisopropyl-3-(2-hydroxy-5-hydroxymethylphenyl)-3-phenylpropylamine
15. 207679-81-0 (free Base)
16. Benzenemethanol, 3-[(1r)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxy-
17. Desfesoterodine (inn)
18. (r)-(+)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethylphenol
19. Benzenemethanol, 3-((1r)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-hydroxy-
20. 2-[(1r)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenol
21. Unii-yu871o78gr
22. Desfesoterodin
23. R-5-hydroxymethyl Tolterodine
24. 5-hm
25. (r)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol
26. 5-hydroxymethyl-tolterodine
27. Intermediate Of Fesoterodine
28. Schembl209062
29. (r)-5-hydroxymethyltolterodine
30. Zinc6047
31. Chembl3348932
32. Desfesoterodine [who-dd]
33. Dtxsid40431319
34. Chebi:177454
35. Dd 01
36. Hms3884p12
37. Bcp02922
38. Spm-7605
39. Mfcd09264524
40. Pnu200577
41. S2659
42. Akos005146249
43. Akos015841720
44. Ccg-267936
45. Cs-0825
46. Db15578
47. Ncgc00346585-01
48. Ac-23943
49. Bs-15813
50. Hy-76569
51. Bcp0726000296
52. 5-hydroxymethyl Tolterodine (pnu 200577)
53. 5-hydroxymethyl Tolterodine - Pnu 200577
54. Sw219846-1
55. D10853
56. F31141
57. 264h524
58. J-013606
59. Q27294711
60. (r)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxy Methyl Phenol
61. (r)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxy Methylphenol
62. (r)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethylphenol
63. (r)-4-hydroxymethyl-2-(3-diisopropylamino-1-phenylpropyl)-phenol
| Molecular Weight | 341.5 g/mol |
|---|---|
| Molecular Formula | C22H31NO2 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 341.235479232 g/mol |
| Monoisotopic Mass | 341.235479232 g/mol |
| Topological Polar Surface Area | 43.7 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 357 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BD - Drugs for urinary frequency and incontinence
G04BD13 - Desfesoterodine
5-Hydroxymethyl tolterodine is a known human metabolite of tolterodine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560