

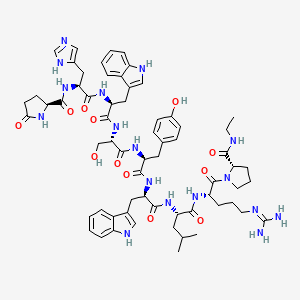

1. 6-trp-10-n-et-glynh2-lhrh
2. D-trp(6)-n-et-d-glynh2(10)-lhrh
3. Deslorelin Acetate
4. Gnrh, Trp(6)-n-et-glynh2(10)-
5. Lhrh, Trp(6)-n-et-glynh2(10)-
6. Lhrh, Tryptophyl(6)-n-ethylglycinamide(10)-
7. Ovuplant
8. Somagard
1. 57773-65-6
2. Somagard
3. D-trp Lhrh-pea
4. D-trp-lhrh-pea
5. Bachem 9022
6. Tkg3i66tve
7. 1-9-luteinizing Hormone-releasing Factor (swine),6-d-tryptophan-9-(n-ethyl-l-prolinamide)-
8. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-tryptophyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide
9. Deslorelina
10. Desloreline
11. Deslorelinum
12. Ncgc00167516-01
13. Unii-tkg3i66tve
14. Desloreline [inn-french]
15. Deslorelinum [inn-latin]
16. Deslorelina [inn-spanish]
17. Gnrh (d-trp6,pro9-net)
18. Deslorelin [usan:inn:ban]
19. (d-trp6,pro9-nhet)lh-rh
20. (d-trp6,des-gly10)-lh-rh Ethylamide
21. (de-gly10,d-trp6,pro-nhet)-lh-rh
22. (des-gly10(d-tro6)-lh-rh Ethylamide
23. Lhrh-t
24. (d-trp(sub 6)-pro(sup 9)-net)-gnrh
25. Deslorelin [mi]
26. Deslorelin [inn]
27. Deslorelin (usan/inn)
28. Deslorelin [usan]
29. (d-trp6,des-gly-nh210)-lh-rh Ethylamide
30. D-trp(sup 6)-pro(sup 9)-n-ethylamide-lhrh
31. (d-trp(sup 6)-pro(sup 9))-lhrh Ethylamide
32. H 4065
33. Deslorelin [mart.]
34. Deslorelin [who-dd]
35. Schembl59413
36. Gtpl9343
37. Chembl2365665
38. Dtxsid2048323
39. Schembl19409316
40. Bdbm84726
41. Chebi:177570
42. (d-trp(sub 6)-pro(sup 9)-net)-gonadotropin Releasing Hormone
43. Gonadotropin Releasing Hormone, (d-trp(sup 6)-pro(sup 9)-net)-
44. Des-gly-10-trp-6-ethylamide-lhrh
45. (d-trp(sup 6)-pro(sup 9))-luteinizing Hormone-releasing Hormone Ethylamide
46. Akos015994649
47. Cs-5746
48. Db11510
49. Hs-2009
50. 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-1-9-luteinizing Hormone-releasing Factor (swine)
51. Gnrh, Trp(6)-n-et-pronh2(9)-
52. Lhrh, Trp(6)-n-et-pronh2(9)-
53. Ncgc00167516-02
54. Ncgc00167516-03
55. Gnrh, Des-gly(10)-trp(6)-ethylamide-
56. Hy-12556
57. Lhrh, Des-gly(10)-trp(6)-ethylamide-
58. 6-trp-9-n-et-pro-10-des-glynh2-lhrh
59. 6-d-phenylalanine-9-(n-ethyl-l-prolinamide)-
60. D03694
61. 773d656
62. Des-gly10,(d-trp6)luteinizing Hormone*releasing H
63. Lhrh, Des-glycyl(10)-tryptophyl(6)-ethylamide-
64. Q5264591
65. (d-trp6-pro9-net)luteinizing Hormone-releasing Factor
66. (d-trp(sup 6),des-gly(sup 10))-lh-rh Ethylamide
67. [des-gly10, D-trp6]-lh-rh Ethylamide, >=97% (hplc)
68. Lhrh, Tryptophyl(6)-n-ethylprolinamide(9)-des-glycinamide(10)-
69. 1-9-luteinizing Hormone-releaasing Factor (swine), 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-
70. Luteinizing Hormone-releasing Factor (pig), 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-10-deglycinamide-
71. Luteinizing Hormone-releasing Factor, 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-10-deglycinamide-
| Molecular Weight | 1282.4 g/mol |
|---|---|
| Molecular Formula | C64H83N17O12 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 32 |
| Exact Mass | 1281.64071115 g/mol |
| Monoisotopic Mass | 1281.64071115 g/mol |
| Topological Polar Surface Area | 447 Ų |
| Heavy Atom Count | 93 |
| Formal Charge | 0 |
| Complexity | 2610 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)