
1. 6-trp-10-n-et-glynh2-lhrh
2. D-trp(6)-n-et-d-glynh2(10)-lhrh
3. Deslorelin
4. Gnrh, Trp(6)-n-et-glynh2(10)-
5. Lhrh, Trp(6)-n-et-glynh2(10)-
6. Lhrh, Tryptophyl(6)-n-ethylglycinamide(10)-
7. Ovuplant
8. Somagard
1. 82318-06-7
2. 82318-06-7 (acetate)
3. 679007nr5c
4. Deslorelin Monoacetate
5. Oxopyrrolidine-2-carboxamide
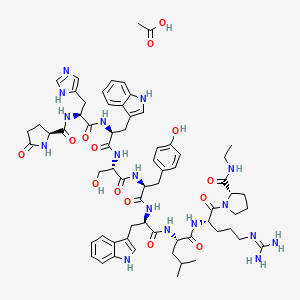
6. (s)-1-((3s,6s,9s,12s,15r,18s,21s)-3-((1h-imidazol-5-yl)methyl)-6,15-bis((1h-indol-3-yl)methyl)-21-(3-((diaminomethylene)amino)propyl)-12-(4-hydroxybenzyl)-9-(hydroxymethyl)-18-isobutyl-1,4,7,10,13,16,19-heptaoxo-1-((s)-5-oxopyrrolidin-2-yl)-2,5,8,11,14,17,20-heptaazadocosan-22-oyl)-n-ethylpyrrolidine-2-carboxamide Acetate
7. Ncgc00167516-01
8. Unii-679007nr5c
9. 1h-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1h-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1h-imidazol-4-yl)-1-oxopropan-2-yl]-5-
10. Acetic Acid;(2s)-n-[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2r)-1-[[(2s)-1-[[(2s)-5-carbamimidamido-1-[(2s)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(
11. Suprelorin
12. Bachem 9022 Acetate
13. Dsstox_cid_26683
14. Dsstox_rid_81818
15. Dsstox_gsid_46683
16. Schembl205614
17. Deslorelin Acetate [mi]
18. Chembl2357192
19. Dtxsid1046683
20. Deslorelin Acetate [who-dd]
21. Tox21_112514
22. Mfcd09842868
23. Akos030485982
24. A73d656
25. Deslorelin Acetate [green Book]
26. Cas-82318-06-7
27. Deslorelin Acetate(57773-65-6 Free Base)
28. Deslorelin Acetate [ema Epar Veterinary]
29. Q27264098
30. (des-gly10,d-trp6,pro-nhet9)-lhrh High Acetate Salt
31. Deslorelin Acetate 100 Microg/ml In Acetonitrile:methanol
32. (d-trp(sup 6),des-gly(sup 10))-lh-rh Ethylamide Acetate
33. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-tryptophyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide Acetate
34. 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-1-9-luteinizing Hormone-releasing Factor (swine) Acetate
35. 6-d-tryptophan-9-(n-ethyl-l-prolinamide)-1-9-luteinizing Hormone-releasing Factor (swine) Monoacetate
36. L-prolinamide, 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-tryptophyl-l-leucyl-l-arginyl-n-ethyl-,?acetate (1:1)
| Molecular Weight | 1342.5 g/mol |
|---|---|
| Molecular Formula | C66H87N17O14 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 32 |
| Exact Mass | 1341.66184052 g/mol |
| Monoisotopic Mass | 1341.66184052 g/mol |
| Topological Polar Surface Area | 485 Ų |
| Heavy Atom Count | 97 |
| Formal Charge | 0 |
| Complexity | 2640 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
For the induction of temporary infertility in healthy, entire, sexually mature male dogs and ferrets.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
QH01CA93