

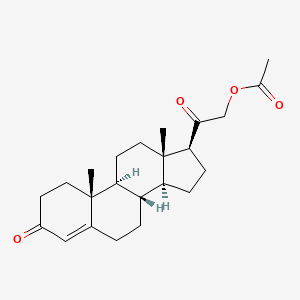

1. Acetate, Desoxycortone
2. Deoxycorticosterone 21 Acetate
3. Deoxycorticosterone-21-acetate
4. Desoxycorticosterone 21 Acetate
5. Desoxycorticosterone Acetate
6. Desoxycorticosterone-21-acetate
7. Desoxycortone Acetate
8. Doc 21 Acetate
9. Doc-21-acetate
10. Doca
11. Syncortyl
1. 56-47-3
2. Desoxycorticosterone Acetate
3. Desoxycortone Acetate
4. Doca
5. Cortexone Acetate
6. Percotol
7. Doc Acetate
8. 11-deoxycorticosterone Acetate
9. Decosteron
10. Decosterone
11. Sincortex
12. Syncortyl
13. Deoxycortone Acetate
14. Descorterone
15. Pregn-4-ene-3,20-dione, 21-(acetyloxy)-
16. Cortacet
17. Cortenil
18. Cortesan
19. Cortifar
20. Cortigen
21. Cortinaq
22. Cortiron
23. Cortivis
24. Cortixyl
25. Decorten
26. Decorton
27. Decostrate
28. Descornaq
29. Desocort
30. Docaquosum
31. Dorcostrin
32. Doxatone
33. Doxycamon
34. Krinocorts
35. Mincortid
36. Ocritena
37. Primocort
38. Primocortan
39. Syncorta
40. Unidocan
41. Arcort
42. Cortate
43. Ocriten
44. Steraq
45. Doca Acetate
46. Bio-corten
47. 3,20-dioxopregn-4-en-21-yl Acetate
48. Doc-ac
49. 21-(acetyloxy)-pregn-4-ene-3,20-dione
50. Organon's Doca Acetate
51. 21-acetoxypregn-4-ene-3,20-dione
52. Percorten
53. Syncort
54. Deoxycorticosterone 21-acetate
55. 11-desoxycorticosterone Acetate
56. Desoxycorticosterone-21-acetate
57. Corticosterone, Deoxy-, Acetate
58. 4-pregnene-3,20-dione-21-ol Acetate
59. 11-deoxycorticosterone 21-acetate
60. 4-pregnene-3,20-dione 21-acetate
61. Doxo
62. 21-acetoxy-3,20-diketopregn-4-ene
63. 21-hydroxypregn-4-ene-3,20-dione 21-acetate
64. Pregn-4-ene-3,20-dione, 21-hydroxy-, Acetate
65. Mls000028509
66. Chebi:34671
67. 6e0a168ob8
68. Desoxycorticosterone Acetate [usp]
69. Nsc-9567
70. Ncgc00022043-04
71. Desoxykorton
72. Cortormon
73. Percortene
74. Smr000058339
75. Dsstox_cid_2894
76. Dsstox_rid_76777
77. Dsstox_gsid_22894
78. Descotone
79. 2-((8s,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl Acetate
80. 11-deoxycorticosterone, Acetate
81. Desoxycorticosterone Acetate (usp)
82. Cas-56-47-3
83. Ccris 7517
84. Nsc 9567
85. Einecs 200-275-9
86. Brn 2570798
87. Unii-6e0a168ob8
88. 21-acetoxypregn-4-en-3,20-dione
89. 21-acetoxy-4-pregnene-3,20-dione
90. 21-acetyloxypregn-4-ene-3,20-dione
91. Doca (tn)
92. Opera_id_47
93. St075186
94. Schembl4169
95. Deoxy Corticosterone Acetate
96. 4-08-00-02195 (beilstein Handbook Reference)
97. Mls001074065
98. Megxm0_000470
99. Chembl1200542
100. Dtxsid6022894
101. Niosh/tu5076000
102. Acon1_002189
103. 11-deoxycorticosterone21-acetate
104. Deoxycorticosterone Acetate, 98%
105. Hms2230b14
106. Hy-b1472
107. Zinc4428527
108. Tox21_110876
109. Mfcd00003660
110. S4243
111. Desoxycortone Acetate [mart.]
112. Akos004910359
113. Akos015837895
114. Desoxycortone Acetate [who-dd]
115. Tox21_110876_1
116. Ccg-268330
117. Cs-5159
118. Db06780
119. Deoxycorticosterone Acetate [mi]
120. Ncgc00022043-05
121. Ncgc00022043-06
122. [2-[(8s,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]-2-oxo-ethyl] Acetate
123. [2-[(8s,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
124. 2-((1s,10s,11s,14s,15s,2r)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0<2,7>.0<11,15> ]heptadec-6-en-14-yl)-2-oxoethyl Acetate
125. As-11731
126. 3,20-dioxopregn-4-en-21-yl Acetate #
127. Desoxycortone Acetate [ep Impurity]
128. 21-hydroxypregn-4-ene-3,20-dione Acetate
129. Desoxycorticosterone Acetate [vandf]
130. D0047
131. Desoxycorticosterone Acetate [usp-rs]
132. Tu50760000
133. D03698
134. D81855
135. Ab00383000_10
136. 003d660
137. A831058
138. Desoxycorticosterone Acetate [orange Book]
139. Desoxycorticosterone Acetate [usp Impurity]
140. Sr-01000000097
141. 21-hydroxypregn-4-ene-3,20-dione Acetate (ester)
142. Sr-01000000097-3
143. W-105514
144. Brd-k24556098-001-01-9
145. Brd-k24556098-001-19-1
146. Q27116214
147. Pregn-4-ene-3,20-dione, 21-hydroxy-, Acetate (ester)
148. Desoxycortone Acetate, European Pharmacopoeia (ep) Reference Standard
149. Deoxycorticosterone Acetate, United States Pharmacopeia (usp) Reference Standard
150. 2-((8s,9s,10r,13s,14s,17s)-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl)-2-oxoethylacetate
151. Acetic Acid [2-keto-2-[(8s,9s,10r,13s,14s,17s)-3-keto-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]ethyl] Ester
| Molecular Weight | 372.5 g/mol |
|---|---|
| Molecular Formula | C23H32O4 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 372.23005950 g/mol |
| Monoisotopic Mass | 372.23005950 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 707 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mineralocorticoids
A group of CORTICOSTEROIDS primarily associated with water and electrolyte balance. This is accomplished through the effect on ION TRANSPORT in renal tubules, resulting in retention of sodium and loss of potassium. Mineralocorticoid secretion is itself regulated by PLASMA VOLUME, serum potassium, and ANGIOTENSIN II. (See all compounds classified as Mineralocorticoids.)