

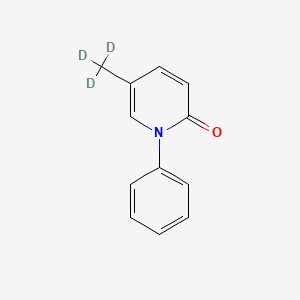

1. 1-phenyl-5-(trideuteriomethyl)-1,2-dihydropyridin-2-one
2. 5-methyl-1-phenyl-2-(1h)-pyridone
3. Deskar
4. Esbriet
5. Pirfenidone
1. 1qc36bhi6s
2. Lyt-100
3. 1093951-85-9
4. 2(1h)-pyridinone, 5-(methyl-d3)-1-phenyl-
5. 1-phenyl-5-(trideuteriomethyl)-1,2-dihydropyridin-2-one
6. Deupirfenidone [usan]
7. Unii-1qc36bhi6s
8. Lyt100
9. Schembl4059454
10. Deupirfenidone [who-dd]
11. Sd-560
12. Who 12460
13. At18410
| Molecular Weight | 188.24 g/mol |
|---|---|
| Molecular Formula | C12H11NO |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 188.102894212 g/mol |
| Monoisotopic Mass | 188.102894212 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 285 |
| Isotope Atom Count | 3 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)