


1. Dextran
2. Dextran 40000
3. Dextran 70
4. Dextran 75
5. Dextran 80
6. Dextran B 1355
7. Dextran B 1355 S
8. Dextran B-1355
9. Dextran B-1355-s
10. Dextran B1355
11. Dextran B512
12. Dextran Derivatives
13. Dextran M 70
14. Dextran T 40
15. Dextran T 500
16. Dextran T 70
17. Dextran T-40
18. Dextran T-500
19. Dextrans
20. Hemodex
21. Hyskon
22. Infukoll
23. Macrodex
24. Polyglucin
25. Promit
26. Rheodextran
27. Rheoisodex
28. Rheomacrodex
29. Rheopolyglucin
30. Rondex
31. Saviosol
1. L3l3xyp7mp
2. Lmwd
3. Schembl206877
4. Chembl1697742
5. O-alpha-d-glucopyranosyl-(1.6)-o-alpha-d-glucopyranosyl-(1.6)-d-glucose
6. Hy-n0913a
7. Dtxsid701317190
8. 6-.alpha.-isomaltosylglucose
9. Zinc64622163
10. Cs-0109495
11. D-glucose, O-.alpha.-d-glucopyranosyl-(1->6)-o-.alpha.-d-glucopyranosyl-(1->6)-
12. O-alpha-d-glucopyranosyl-(1-->6)-o-alpha-d-glucopyranosyl-(1-->6)-d-glucose
13. Wurcs=2.0/2,3,2/[o2122h][a2122h-1a_1-5]/1-2-2/a6-b1_b6-c1
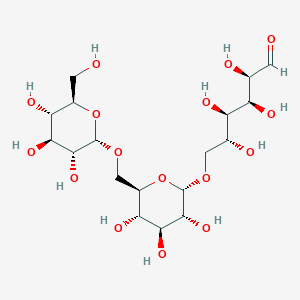
14. (2r,3s,4r,5r)-2,3,4,5-tetrahydroxy-6-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl)oxy)methyl)oxan-2-yl)oxy)hexanal
| Molecular Weight | 504.4 g/mol |
|---|---|
| Molecular Formula | C18H32O16 |
| XLogP3 | -7.2 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 11 |
| Exact Mass | 504.16903493 g/mol |
| Monoisotopic Mass | 504.16903493 g/mol |
| Topological Polar Surface Area | 277 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 625 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Plasma Substitutes
Any liquid used to replace blood plasma, usually a saline solution, often with serum albumins, dextrans or other preparations. These substances do not enhance the oxygen- carrying capacity of blood, but merely replace the volume. They are also used to treat dehydration. (See all compounds classified as Plasma Substitutes.)