


1. Hyperstat
2. Proglycem
1. 364-98-7
2. Proglycem
3. Hyperstat
4. Hypertonalum
5. Eudemine
6. Proglicem
7. Dizoxide
8. Mutabase
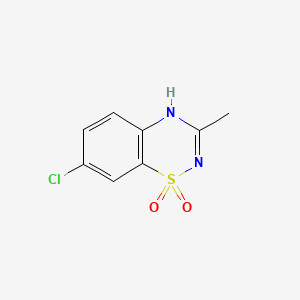
9. 7-chloro-3-methyl-2h-1,2,4-benzothiadiazine 1,1-dioxide
10. Sch 6783
11. Sch-6783
12. Diazossido
13. Diazoxidum
14. Srg 95213
15. Srg-95213
16. Diazoxido
17. 2h-1,2,4-benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide
18. Eudemine Injection
19. Nsc-64198
20. Nsc 76130
21. Chebi:4495
22. Mfcd00078578
23. Nsc-76130
24. O5cb12l4fn
25. 3-methyl-7-chloro-1,2,4-benzothiadiazine 1,1-dioxide
26. Mls000028459
27. 7-cloro-3-metil-2h-1,2,4-benzotiodiazina-1,1-diossido
28. 364-98-7 (free)
29. 7-chloro-3-methyl-4h-1$l^{6},2,4-benzothiadiazine 1,1-dioxide
30. Diazossido [italian]
31. Nsc64198
32. Nsc76130
33. 7-chloro-3-methyl-2h-1,2,4-benzothiadiazine1,1-dioxide
34. C8h7cln2o2s
35. Diazossido [dcit]
36. Ncgc00015380-09
37. Cas-364-98-7
38. Smr000058392
39. 7-chloro-3-methyl-2h-benzo[e][1,2,4]thiadiazine 1,1-dioxide
40. Dsstox_cid_2914
41. Diazoxidum [inn-latin]
42. Dsstox_rid_76786
43. Diazoxido [inn-spanish]
44. Dsstox_gsid_22914
45. 7-chloro-3-methyl-4h-1,2,4-benzothiadiazine 1,1-dioxide
46. Aroglycem
47. 7-chloro-3-methyl-4h-benzo[e][1,2,4]thiadiazine 1,1-dioxide
48. Hyperstat (tn)
49. Sr-01000075314
50. Einecs 206-668-1
51. Nsc 64198
52. Unii-o5cb12l4fn
53. 7-chloro-3-methyl-2h-1,4-benzothiadiazine 1,1-dioxide
54. Diazoxide (jan/usp/inn)
55. Eudimine
56. 2h-1,4-benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide
57. 7-chloro-3-methyl-2h-1?^{6},2,4-benzothiadiazine 1,1-dioxide
58. 7-cloro-3-metil-2h-1,2,4-benzotiodiazina-1,1-diossido [italian]
59. Proglycem (tn)
60. Prestwick_163
61. Diazoxide [usan:usp:inn:ban:jan]
62. Tocris-0964
63. Diazoxide [inn]
64. Diazoxide [jan]
65. Opera_id_608
66. Diazoxide [mi]
67. Diazoxide [usan]
68. Prestwick0_000087
69. Prestwick1_000087
70. Prestwick2_000087
71. Prestwick3_000087
72. Spectrum3_000735
73. Spectrum4_001248
74. Diazoxide [vandf]
75. Lopac-d-9035
76. Chembl181
77. Diazoxide [mart.]
78. D 9035
79. Diazoxide [usp-rs]
80. Diazoxide [who-dd]
81. Diazoxide [who-ip]
82. Cbiol_001750
83. Lopac0_000404
84. Schembl41254
85. Bspbio_000014
86. Bspbio_001307
87. Bspbio_002290
88. Kbiogr_000027
89. Kbiogr_001776
90. Kbioss_000027
91. Mls001076071
92. Mls001424164
93. Spectrum2300206
94. Spbio_001953
95. Bpbio1_000016
96. Gtpl2409
97. Diazoxide [orange Book]
98. Chembl1518123
99. Diazoxide [ep Monograph]
100. Dtxsid7022914
101. Bdbm86248
102. Kbio2_000027
103. Kbio2_002595
104. Kbio2_005163
105. Kbio3_000053
106. Kbio3_000054
107. Kbio3_001510
108. Diazoxide [usp Monograph]
109. Diazoxidum [who-ip Latin]
110. Bio1_000036
111. Bio1_000525
112. Bio1_001014
113. Bio2_000027
114. Bio2_000507
115. Hms1361b09
116. Hms1568a16
117. Hms1791b09
118. Hms1922l22
119. Hms1989b09
120. Hms2051p20
121. Hms2089l04
122. Hms2093n12
123. Hms2095a16
124. Hms2234b23
125. Hms3261a10
126. Hms3267i11
127. Hms3371l13
128. Hms3393p20
129. Hms3402b09
130. Hms3411l18
131. Hms3675l18
132. Hms3712a16
133. Hms3885h12
134. Pharmakon1600-02300206
135. 2h-1,2, 4-benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide
136. 7-chloro-3-methyl-1lambda~4~,2,4-benzothiadiazin-1-ol 1-oxide
137. 7-chloro-3-methyl-4h-1lambda6,2,4-benzothiadiazine 1,1-dioxide
138. Bcp26107
139. Hy-b1140
140. Nsc_3019
141. Zinc3872277
142. Tox21_110132
143. Tox21_500404
144. Bdbm50237612
145. Kc-115
146. Nsc759574
147. S4630
148. Akos015896340
149. Akos024458715
150. Tox21_110132_1
151. Ccg-101062
152. Ccg-204497
153. Cs-4745
154. Db01119
155. Ks-1444
156. Lp00404
157. Nc00312
158. Nsc-759574
159. Sdccgsbi-0050390.p002
160. Idi1_033777
161. Diazoxide 100 Microg/ml In Acetonitrile
162. Ncgc00015380-01
163. Ncgc00015380-02
164. Ncgc00015380-03
165. Ncgc00015380-04
166. Ncgc00015380-05
167. Ncgc00015380-06
168. Ncgc00015380-07
169. Ncgc00015380-08
170. Ncgc00015380-10
171. Ncgc00015380-11
172. Ncgc00015380-12
173. Ncgc00015380-13
174. Ncgc00015380-20
175. Ncgc00022882-03
176. Ncgc00024907-01
177. Ncgc00024907-02
178. Ncgc00024907-03
179. Ncgc00024907-04
180. Ncgc00024907-05
181. Ncgc00024907-06
182. Ncgc00024907-07
183. Ncgc00024907-08
184. Ncgc00261089-01
185. Cas_364-98-7
186. Sy066792
187. Db-048966
188. B6526
189. D5402
190. Eu-0100404
191. Ft-0603087
192. Vu0239714-6
193. C06949
194. D00294
195. F12855
196. 364d987
197. A823275
198. Q420009
199. Sr-01000075314-1
200. Sr-01000075314-3
201. Sr-01000075314-4
202. Sr-01000075314-6
203. 3-methyl-7-chloro-1,2,4-benzothiadiazine1,1-dioxide
204. Brd-k73109821-001-05-2
205. Brd-k73109821-001-10-2
206. Diazoxide, European Pharmacopoeia (ep) Reference Standard
207. 4h-1,2,4-benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide
208. 7-chloro-3-methyl-4h-1
209. E?,2,4-benzothiadiazine-1,1-dione
210. 7-chloro-3-methyl-4h-1
211. E6,2,4-benzothiadiazine 1,1-dioxide
212. Diazoxide, United States Pharmacopeia (usp) Reference Standard
213. 7-chloro-3-methyl-2h-1$l^{6},2,4-benzothiadiazine 1,1-dioxide
214. 7-chloro-3-methyl-4h-1$l^{6},2,4-benzothiadiazine-1,1-dione
| Molecular Weight | 230.67 g/mol |
|---|---|
| Molecular Formula | C8H7ClN2O2S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 229.9916763 g/mol |
| Monoisotopic Mass | 229.9916763 g/mol |
| Topological Polar Surface Area | 66.9 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 360 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Proglycem |
| PubMed Health | Diazoxide (By mouth) |
| Drug Classes | Gastrointestinal Agent, Glucose Regulation, Antihypoglycemic |
| Drug Label | PROGLYCEM (diazoxide) is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. PROGLYCEM Capsules contain 50 mg diazoxide, USP. The Suspension contains 50 mg of diazoxide, USP in each milliliter an... |
| Active Ingredient | Diazoxide |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Proglycem |
| PubMed Health | Diazoxide (By mouth) |
| Drug Classes | Gastrointestinal Agent, Glucose Regulation, Antihypoglycemic |
| Drug Label | PROGLYCEM (diazoxide) is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. PROGLYCEM Capsules contain 50 mg diazoxide, USP. The Suspension contains 50 mg of diazoxide, USP in each milliliter an... |
| Active Ingredient | Diazoxide |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
Used parentally to treat hypertensive emergencies. Also used to treat hypoglycemia secondary to insulinoma.
Diazoxide is a potassium channel activator. Its mechanism of action revolves around enhancing cell membrane permeability to potassium ions. This action consequently elicits the relaxation of local smooth muscles. This switches off voltage-gated calcium ion channels which inhibits the generation of an action potential.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C02 - Antihypertensives
C02D - Arteriolar smooth muscle, agents acting on
C02DA - Thiazide derivatives
C02DA01 - Diazoxide
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AH - Drugs for treatment of hypoglycemia
V03AH01 - Diazoxide
Absorption
Readily absorbed following oral administration.
Route of Elimination
Proglycem is extensively bound (more than 90%) to serum proteins, and is excreted in the kidneys.
Hepatic.
28 ±8.3 hours in normal adults.
Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance.
