
1. Bishydroxycoumarin
2. Dicoumarin
3. Dicoumarol
1. Dicoumarol
2. 66-76-2
3. Bishydroxycoumarin
4. Dicoumarin
5. Melitoxin
6. Bis-hydroxycoumarin
7. Antitrombosin
8. Baracoumin
9. Dicoumal
10. Dicumarine
11. Acadyl
12. Acavyl
13. Dicuman
14. Dicumol
15. Trombosan
16. Dufalone
17. Kumoran
18. Temparin
19. Cumid
20. Cuma
21. Dicumaol R
22. Dicoumarolum
23. 3,3'-methylenebis(4-hydroxycoumarin)
24. Bis(4-hydroxycoumarin-3-yl)methane
25. Bis-3,3'-(4-hydroxycoumarinyl)methane
26. Di-(4-hydroxy-3-coumarinyl)methane
27. Dicumarol [usan]
28. Di-4-hydroxy-3,3'-methylenedicoumarin
29. 3,3'-methylen-bis(4-hydroxy-cumarin)
30. 3,3'-methylenebis(4-hydroxy-2h-1-benzopyran-2-one)
31. 3,3'-methylene-bis(4-hydroxycoumarine)
32. 3,3'-methylenebis(4-hydroxy-1,2-benzopyrone)
33. 3,3'-methylenebis(4-hydroxy-2h-chromen-2-one)
34. Nc 034
35. 2h-1-benzopyran-2-one, 3,3'-methylenebis[4-hydroxy-
36. 3,3'-metilen-bis(4-idrossi-cumarina)
37. 3,3'-methyleen-bis(4-hydroxy-cumarine)
38. 3,3'-methanediylbis(4-hydroxy-2h-chromen-2-one)
39. Nsc 17860
40. Dicoumarol (inn)
41. Dicoumarol [inn]
42. 3,3'-methylenebis[4-hydroxycoumarin]
43. Dicumarol (usan)
44. Nsc 221570
45. 3,3'-methylene-bis(4-hydroxycoumarin)
46. 4-hydroxy-3-[(4-hydroxy-2-oxochromen-3-yl)methyl]chromen-2-one
47. Coumarin, 3,3'-methylenebis(4-hydroxy-
48. Nsc-17860
49. 7qid3e7bg7
50. Chembl1466
51. 2h-1-benzopyran-2-one), 3,3'-methylenebis(4-hydroxy-
52. Chebi:4513
53. Anathrombase
54. Apekumarol
55. Dicoumerol
56. Dicumarinum
57. Dicumarolum
58. 4-hydroxy-3-[(4-hydroxy-2-oxo-2h-chromen-3-yl)methyl]-2h-chromen-2-one
59. Nsc17860
60. Nsc41834
61. 3,2-benzopyrone]
62. Nsc-221570
63. Cas-66-76-2
64. Dicumarolo [dcit]
65. Ncgc00016296-01
66. Dwukumarol [polish]
67. Dicumarolo
68. Dikumarol
69. Dwukumarol
70. Dsstox_cid_1729
71. 3,3'-methylenebis[4-hydroxy-1,2-benzopyrone]
72. Dsstox_rid_76296
73. Dicumarol [inn-spanish]
74. Dsstox_gsid_21729
75. Dicoumarolum [inn-latin]
76. Chembl43154
77. 3,3'-methylenebis[4-hydroxy-2h-1-benzopyran-2-one]
78. 2h-1-benzopyran-2-one, 3,3'-methylenebis(4-hydroxy-
79. Coumarin,3'-methylenebis[4-hydroxy-
80. Dicumarol (tn)
81. Ccris 3713
82. Bis-3,3'-(4-oxycoumarinyl)ethylacetate
83. Nsc221570
84. Hsdb 3223
85. Wln: T66 Bovj Eq D1- Dt66 Bovj Eq
86. Sr-05000001605
87. 4,4'-dihydroxy-3,3'-methylene Bis Coumarin
88. Dicumarol [usan:usp]
89. 2h-1-benzopyran-2-one,3'-methylenebis[4-hydroxy-
90. Einecs 200-632-9
91. Nsc 41834
92. 2h-1-benzopyran-2-one],3'-methylenebis[4-hydroxy-
93. 3,3'-methylen-bis(4-hydroxy-cumarin) [german]
94. 3,3'-methyleen-bis(4-hydroxy-cumarine) [dutch]
95. 3,3'-methylene-bis(4-hydroxycoumarine) [french]
96. 3,3'-metilen-bis(4-idrossi-cumarina) [italian]
97. Unii-7qid3e7bg7
98. Brn 0335444
99. Ai3-14546
100. 4-hydroxy-3-((4-hydroxy-2-oxo-2h-chromen-3-yl)methyl)-2h-chromen-2-one
101. Symmetric Dicoumarol Analogue, 1
102. Uncoupler Of Oxidative Respiration
103. Prestwick_90
104. Mfcd00006857
105. Ethylm-trifluoromethylcarbanilate
106. Spectrum_000165
107. Dicumarol [mi]
108. Dicumarol [hsdb]
109. Prestwick0_000785
110. Prestwick1_000785
111. Prestwick2_000785
112. Prestwick3_000785
113. Spectrum2_000144
114. Spectrum3_000387
115. Spectrum4_000508
116. Spectrum5_000871
117. Dicumarol [vandf]
118. M0216
119. Chemdiv2_003436
120. Dicoumarol [mart.]
121. Dicoumarol [who-dd]
122. Dicoumarol [who-ip]
123. Oprea1_150990
124. Schembl33891
125. Schembl33892
126. Bspbio_000890
127. Bspbio_002173
128. Cbdive_003005
129. Kbiogr_001055
130. Kbioss_000645
131. 5-19-06-00682 (beilstein Handbook Reference)
132. Divk1c_000896
133. Spectrum1500239
134. Spbio_000248
135. Spbio_002829
136. Bpbio1_000980
137. Gtpl6808
138. Dicumarol [orange Book]
139. Dtxsid8021729
140. Bdbm35525
141. Hms502m18
142. Kbio1_000896
143. Kbio2_000645
144. Kbio2_003213
145. Kbio2_005781
146. Kbio3_001393
147. Dicoumarol - Cas 66-76-2
148. Ninds_000896
149. Hms1378m04
150. Hms1570m12
151. Hms1920e20
152. Hms2091m10
153. Hms2097m12
154. Hms3652p10
155. Hms3714m12
156. Hms3744m19
157. Hms3865f03
158. Pharmakon1600-01500239
159. Dicoumarolum [who-ip Latin]
160. Hy-n0645
161. Zinc3869855
162. Tox21_110357
163. 3,3'-methylenbis(4-hydroxycumarin)
164. 3,3-methylenebis(4-hydroxycoumarin)
165. Bbl008904
166. Ccg-34550
167. Nsc756733
168. S4299
169. Stk801287
170. Akos000520650
171. Tox21_110357_1
172. Am10016
173. Cs-7962
174. Db00266
175. Nsc-756733
176. Idi1_000896
177. Ncgc00016296-02
178. Ncgc00016296-03
179. Ncgc00016296-04
180. Ncgc00016296-05
181. Ncgc00016296-07
182. Ncgc00094650-01
183. Ncgc00094650-02
184. As-19619
185. Sbi-0051343.p003
186. Ft-0624734
187. Sw196402-3
188. 3,3''''''''-methylenebis[4-hydroxycoumarin
189. 3,3'-methylene-bis(4-hydroxycoumarin), 99%
190. A14241
191. C00796
192. D03798
193. D91292
194. 3,3''''''''-methylenebis(4-hydroxy-coumarin
195. 3,3''''''''-methylenebis(4-hydroxycoumarin)
196. Ab00051966_05
197. Coumarin, 3,3'-methylenebis[4-hydroxy- (8ci)
198. Q420886
199. Sr-05000001605-1
200. Sr-05000001605-3
201. Sr-05000001605-4
202. W-203471
203. Brd-k82236179-001-05-0
204. Brd-k82236179-001-06-8
205. Z57170530
206. 2h-1-benzopyran-2-one, 3,3'-methylenebis[4-hydroxy- (9ci)
207. Dicumarol, United States Pharmacopeia (usp) Reference Standard
208. 4-hydroxy-3-[(4-hydroxy-2-oxo-chromen-3-yl)methyl]chromen-2-one
| Molecular Weight | 336.3 g/mol |
|---|---|
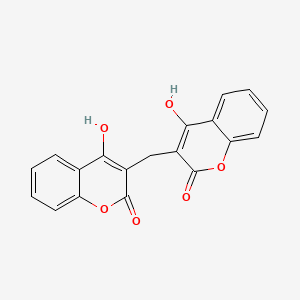
| Molecular Formula | C19H12O6 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 336.06338810 g/mol |
| Monoisotopic Mass | 336.06338810 g/mol |
| Topological Polar Surface Area | 93.1 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 605 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticoagulants; Enzyme Inhibitors; Uncoupling Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Anticoagulants are indicated for prophylaxis and/or treatment of venous (or arterial) thrombosis (and its extension) and pulmonary embolism /Not included in US product labeling/, deep vein thrombosis (DVT) or pulmonary embolism (treatment). Oral anticoagulants are used during and following initial heparin therapy to decrease the risk of extension, recurrence, or death. /Anticoagulants; Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 265
Oral anticoagulants are used to prevent thromboembolic complications after surgery, although low-dose subcutaneous heparin is used more commonly. /Anticoagulants; Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 265
Anticoagulants are indicated for prophylaxis and/or treatment of thromboembolic complications (ischemic stroke) associated with atrial fibrillation. They are strongly recommended in patients at high risk of stroke (including patients with recent stroke, transient ischemic attack, or systemic embolism; poor left ventricular function; age over 75 years; hypertension; rheumatic mitral valve disease; mechanical or tissue prosthetic heart valves.) /Anticoagulants; Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 265
For more Therapeutic Uses (Complete) data for DICUMAROL (9 total), please visit the HSDB record page.
Contraindications to oral anticoagulants include pre-existing or coexisting abnormalities of blood coagulation, active bleeding, recent or imminent surgery of the central nervous system or eye, diagnostic or therapeutic procedures with potential for uncontrollable bleeding including lumbar puncture, malignant hypertension, peptic ulceration, pregnancy, threatened abortion, intrauterine device, cerebrovascular hemorrhage, and bacterial endocarditis. Relative contraindications include thrombocytopenia, pericarditis, pericardial effusions, and unreliability of the patient or of patient supervision. /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 308
Most commonly, oral anticoagulant-induced bleeding is minor and consists of bruising, hematuria, epistaxis, conjunctival hemorrhage, minor gastrointestinal bleeding, bleeding from wounds and sites of trauma, and vaginal bleeding. More serious major or fatal bleeding is most commonly gastrointestinal, intracranial, vaginal, retroperitoneal, or related to a wound or site of trauma, although a large variety of other sites of bleeding have been reported. Intracranial bleeding occurs most frequently in patients receiving oral anticoagulants for cerebrovascular disease and most commonly presents as a subdural hematoma, often unassociated with head trauma. Fatal gastrointestinal bleeding is most commonly from a peptic ulcer, although any gastrointestinal lesion may be a potential source of major bleeding. Overall, a bleeding lesion can be identified in about two thirds of cases of oral anticoagulants-related hemorrhage. /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 311
Overall, the bleeding rate of oral anticoagulant therapy is influenced by several factors: the intensity of anticoagulation, either intentionally or inadvertent; the underlying clinical disorder for which anticoagulant therapy is used (with bleeding occurring most frequently in ischemic cerebrovascular disease and venous thromboembolism; and, with bleeding occurring most commonly in the elderly; the presence of adverse drug interactions or comorbid factors such as clinical states potentiating warfarin action, pre-existing hemorrhagic diathesis, malignancy, recent surgery, trauma, or pre-existing potential bleeding sites (e.g., surgical wound, peptic ulcer, recent cerebral hemorrhage, carcinoma of colon); the simultaneous use of aspirin (but not of dipyridamole); and patient reliability (e.g., increased bleeding in alcoholics not due to ethanol-warfarin drug interaction but rather to unreliability of drug intake). /Oral anticoagulants/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 310
Spontaneous abortion and stillbirth have occurred, as well as low birth weight and growth retardation. In addition, fetal or neonatal hemorrhage, fetal death from hemorrhage in utero, and increased risk of maternal hemorrhage during the second and third trimesters have been reported. There is some evidence that embryopathy occurs only with oral anticoagulant administration between the 6th and 12th weeks of gestation. /Anticoagulants/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 267
For more Drug Warnings (Complete) data for DICUMAROL (34 total), please visit the HSDB record page.
For decreasing blood clotting. Often used along with heparin for treatment of deep vein thrombosis.
Dicumarol is an coumarin-like compound found in sweet clover. It is used as an oral anticoagulant and acts by inhibiting the hepatic synthesis of vitamin K-dependent coagulation factors (prothrombin and factors VII, IX, and X).
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Uncoupling Agents
Chemical agents that uncouple oxidation from phosphorylation in the metabolic cycle so that ATP synthesis does not occur. Included here are those IONOPHORES that disrupt electron transfer by short-circuiting the proton gradient across mitochondrial membranes. (See all compounds classified as Uncoupling Agents.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AA - Vitamin k antagonists
B01AA01 - Dicoumarol
Considerable individual variation in t/2 of dicumarol has been attributed to genetic factors. ... dicumarol...hydroxylated to inactive compounds by enzymes of hepatic endoplasmic reticulum. These metabolites and traces of parent drugs are excreted in urine. Some unabsorbed dicumarol appears in feces.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1359
In man, absorption of dicumarol from gi tract is slow and erratic. ... There is considerable variation in absorption from one individual to another. Within circulation...almost entirely but loosely bound to plasma albumin, & only small percentage of total plasma concentration is represented by unbound drug. ... Appreciable amount ...found in erythrocytes, but little or none is present in cerebrospinal fluid. ...accumulate/s/ mainly in lung, liver, spleen and kidney.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1358
Whole-body autoradiography of rats given anticoagulant, [(14)C]-dicumarol by intracardiac injection, indicated that (14)C distributed in most tissues, maximally in liver, lungs, heart, and kidneys. After 24 hr, (14)C levels were high in intestinal tract owing, presumably, to biliary excretion. Initially, iv dose of dicoumarol was more readily excreted in bile than in urine; in 3 hr, 4% was eliminated in bile and less than 0.4% in urine.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 74
...71% of iv dose of...[(14)C]-dicoumarol, was excreted in feces and 23% in urine in 5 days.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 43
Dicoumarol is not conjugated in either man or dog...
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 151
Dicumarol...hydroxylated to inactive cmpd by enzymes of hepatic endoplasmic reticulum.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1359
Despite their structural similarity to coumarin, the anticoagulants dicumarol and warfarin do not appear to be substrates for CYP2A6. The overall rate of dicumarol metabolism varied approx 5 fold among the human liver microsomal samples, but this variation correlated poorly (r2= 0.126) with the variation observed in CYP2A6 levels and hydroxycoumarin levels.
PMID:1381906 Pearce R et al; Arch Biochem Biophys 298 (1): 211-25 (1992)
1-2 days
...T/2 of dicumarol is dose dependent, ranging from 10 hr at low dosage to 30 hr at high dosage.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1358
Dicumarol has a dose dependent plasma half-life (one to two days); therapy is therefore somewhat difficult to control and frequent monitoring is usually indicated.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 742
Elimination half-life: 1 to 2 days
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 266
Dicumarol inhibits vitamin K reductase, resulting in depletion of the reduced form of vitamin K (vitamin KH2). As vitamin K is a cofactor for the carboxylation of glutamate residues on the N-terminal regions of vitamin K-dependent proteins, this limits the gamma-carboxylation and subsequent activation of the vitamin K-dependent coagulant proteins. The synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S is inhibited. Depression of three of the four vitamin K-dependent coagulation factors (factors II, VII, and X) results in decresed prothrombin levels and a decrease in the amount of thrombin generated and bound to fibrin. This reduces the thrombogenicity of clots.
These compounds depress the hepatic synthesis of vitamin K1-dependent clotting factors (II, VII, IX, X) by inhibiting the vitamin K1 2,3-reductase enzyme in the vitamin K1-epoxide cycle. /Anticoagulant rodenticides/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 1084
The oral anticoagulants block the regeneration of reduced vitamin K and thereby induce a state of functional vitamin K deficiency. The mechanism of the inhibition of reductase(s) by the coumarin drugs is not known. There exist reductases that are less sensitive to these drugs but that act only at relatively high concentrations of oxidized vitamin K; this property may explain the observation that administration of sufficient vitamin K can counteract even large doses of oral anticoagulants. /Oral Anticoagulants/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1527
Dicumarol a specific and potent inhibitor of DT-diaphorase.
PMID:2455523 Atallah AS et al; Biochem Pharmacol 37 (12): 2451-9 (1988)
4-Hydroxycoumarin deriv (dicumarol...) Inhibit rat liver /drug metabolizing enzymes/ in vitro. Mechanism is complex, being caused by several factors, including conversion of cytochrome P450 into P420, inhibition of NADPH-cytochrome P450 reductase, and competition for active site.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 496
For more Mechanism of Action (Complete) data for DICUMAROL (7 total), please visit the HSDB record page.