


1. Carbamazine
2. Citrate, Diethylcarbamazine
3. Diethylcarbamazine Citrate
4. Diethylcarbamazine Citrate (1:1)
5. Diethylcarbamazine Citrate (1:2)
6. Diethylcarbamazine L-tartrate (1:1)
7. Diethylcarbamazine Maleate
8. Diethylcarbamazine Monohydrochloride
9. Diethylcarbamazine Phosphate (1:1)
10. Hetrazan
11. Loxuran
12. Maleate, Diethylcarbamazine
13. Monohydrochloride, Diethylcarbamazine
14. Notezine
1. 90-89-1
2. N,n-diethyl-4-methylpiperazine-1-carboxamide
3. Carbamazine
4. Diethyl Carbamazine
5. Carbilazine
6. Ethodryl
7. Notezine
8. Cypip
9. Bitirazine
10. Caricide
11. Ditrazine Base
12. Caracide
13. Spatonin
14. N,n-diethyl-4-methyl-1-piperazinecarboxamide
15. N,n-diethylcarbamazine
16. 1-piperazinecarboxamide, N,n-diethyl-4-methyl-
17. Camin
18. Rp 3799
19. Diethylcarbamazine (inn)
20. 84l
21. Chebi:4527
22. V867q8x3zd
23. Bitirazine; Caracide;carbamazine
24. Mmv002816
25. Diaethylcarbamazinum
26. Dietilcarbamazina
27. Diethylcarbamazinum
28. Diethylcarbamazine [inn]
29. Diethylcarbamazine [inn:ban]
30. Diethylcarbamazinum [inn-latin]
31. Dietilcarbamazina [inn-spanish]
32. Nsc1364
33. Camin (tn)
34. Einecs 202-023-3
35. 1-diethylcarbamoyl-4-methylpiperazine
36. Brn 0143029
37. Unii-v867q8x3zd
38. Ai3-19612
39. 1-diethylcarbamyl-4-methylpiperazine
40. 1-methyl-4-diethylcarbamoylpiperazine
41. Banocide (salt/mix)
42. Caritrol (salt/mix)
43. Nemacide (salt/mix)
44. Spectrum_000938
45. Nn-diethyl-4-methyl-1-piperazinecarboxamide
46. Prestwick0_000284
47. Prestwick1_000284
48. Prestwick2_000284
49. Prestwick3_000284
50. Spectrum2_001022
51. Spectrum3_000390
52. Spectrum4_000511
53. Spectrum5_000877
54. Diethyl Carbamazine Citrate
55. Chembl684
56. Ec 202-023-3
57. Schembl67289
58. Bspbio_000188
59. Bspbio_002179
60. Kbiogr_001081
61. Kbioss_001418
62. 4-23-00-00225 (beilstein Handbook Reference)
63. Divk1c_000548
64. Spbio_001203
65. Spbio_002407
66. Diethylcarbamazine [mi]
67. Bpbio1_000208
68. Zinc1288
69. Dtxsid1022928
70. Kbio1_000548
71. Kbio2_001418
72. Kbio2_003986
73. Kbio2_006554
74. Kbio3_001399
75. Ninds_000548
76. Hms3604f12
77. Diethylcarbamazine [who-dd]
78. 1-diethylcarbamoyl-4-methylpiperzine
79. Hy-12642a
80. Mfcd00023288
81. Akos003268016
82. Db00711
83. Ds-9360
84. Idi1_000548
85. S10791
86. Ncgc00178778-01
87. Ncgc00178778-02
88. Ncgc00178778-07
89. Sbi-0051345.p003
90. Db-080764
91. R.p. 3799
92. 1-(n,n-diethylcarbamoyl)-4-methylpiperazine
93. Ab00053457
94. Cs-0013568
95. Ft-0624832
96. C07968
97. D07825
98. N,n-diethyl-4-methyl-piperazine-1-carboxamide
99. Ab00053457_12
100. Q409267
101. Sr-01000759234-8
102. Brd-k45542189-048-05-6
103. Brd-k45542189-048-15-5
| Molecular Weight | 199.29 g/mol |
|---|---|
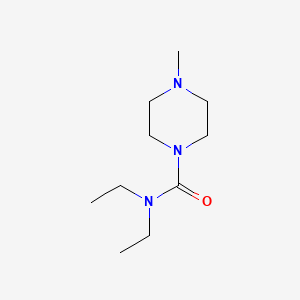
| Molecular Formula | C10H21N3O |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 199.168462302 g/mol |
| Monoisotopic Mass | 199.168462302 g/mol |
| Topological Polar Surface Area | 26.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 184 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the treatment of certain filarial diseases, including tropical pulmonary eosinophilia, loiasis, and lymphatic filariasis caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori.
Diethylcarbamazine is an anthelmintic drug that does not resemble other antiparasitic compounds. It is a synthetic organic compound which is highly specific for several parasites and does not contain any toxic metallic elements.
Lipoxygenase Inhibitors
Compounds that bind to and inhibit that enzymatic activity of LIPOXYGENASES. Included under this category are inhibitors that are specific for lipoxygenase subtypes and act to reduce the production of LEUKOTRIENES. (See all compounds classified as Lipoxygenase Inhibitors.)
Filaricides
Pharmacological agents destructive to nematodes in the superfamily Filarioidea. (See all compounds classified as Filaricides.)
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02C - Antinematodal agents
P02CB - Piperazine and derivatives
P02CB02 - Diethylcarbamazine
Absorption
Readily absorbed following oral administration.
Partially metabolized to diethylcarbamazine N-oxide.
Approximately 8 hours.
The mechanism of action of diethylcarbamazine is thought to involve sensitizing the microfilariae to phagocytosis. One study showed that diethylcarbamazine's activity against Brugia malayi microfilariae is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway. It confirmed the important role of the arachidonic acid metabolic pathway in diethylcarbamazine's mechanism of action in vivo and showes that in addition to its effects on the 5-lipoxygenase pathway, it targets the cyclooxygenase pathway and COX-1.