
1. Carbamazine
2. Citrate, Diethylcarbamazine
3. Diethylcarbamazine
4. Diethylcarbamazine Citrate (1:1)
5. Diethylcarbamazine Citrate (1:2)
6. Diethylcarbamazine L-tartrate (1:1)
7. Diethylcarbamazine Maleate
8. Diethylcarbamazine Monohydrochloride
9. Diethylcarbamazine Phosphate (1:1)
10. Hetrazan
11. Loxuran
12. Maleate, Diethylcarbamazine
13. Monohydrochloride, Diethylcarbamazine
14. Notezine
1. 1642-54-2
2. Hetrazan
3. Loxuran
4. Dicarocide
5. Caritrol
6. Diethylcarbamazine (citrate)
7. Eosinopin
8. Filazine
9. Franocide
10. Franozan
11. Carbam Palatabs
12. Ditrazin Citrate
13. Ethodryl Citrate
14. Diro-form
15. Dec Chewable
16. Dek-tabs
17. Diethylcarbamazine Dihydrogen Citrate
18. Diethylcarbamazane Citrate
19. D.e.c. Sol
20. Diethylcarbamazine Acid Citrate
21. Diethylcarbamazine Citrate Salt
22. Diethylcarbamazine Hydrogen Citrate
23. Ethylaminoazine Citrate
24. 1-methyl-4-diethylcarbamoylpiperazine Citrate
25. Nsc-80513
26. N,n-diethyl-4-methyl-1-piperazinecarboxamide Citrate
27. 1-diethylcarbamoyl-4-methylpiperazine Dihydrogen Citrate
28. Mls000069762
29. Mls001074102
30. Os1z389k8s
31. N,n-diethyl-4-methyl-1-piperazinecarboxamide Citrate (1:1)
32. N,n-diethyl-4-methyl-1-piperazinecarboxamide Dihydrogen Citrate
33. 1-piperazinecarboxamide, N,n-diethyl-4-methyl-, 2-hydroxy-1,2,3-propanetricarboxylate
34. N,n-diethyl-4-methylpiperazine-1-carboxamide 2-hydroxypropane-1,2,3-tricarboxylate
35. Ncgc00017034-01
36. Dirocide
37. Ditrazine
38. Ditrazinum
39. Filarabits
40. Longicid
41. Smr000058650
42. Cas-1642-54-2
43. Ditrazini Citras
44. Carricide Citrate
45. Ditrazine Citrate
46. Dsstox_cid_25555
47. Dsstox_rid_80952
48. N,n-diethyl-4-methylpiperazine-1-carboxamide;2-hydroxypropane-1,2,3-tricarboxylic Acid
49. 1642-54-1(citrate); 90-89-1(free Base)
50. Dsstox_gsid_45555
51. N,n-diethyl-4-methylpiperazine-1-carboxamide Citrate
52. Diethylcarbamazini Citras
53. Hsdb 6421
54. Sr-01000759234
55. Einecs 216-696-6
56. Mfcd00039124
57. Nsc 80513
58. Unii-os1z389k8s
59. Hetrazan (tn)
60. Prestwick_166
61. Diethylcarbamazine Citrate [usp:jan]
62. N,n-diethyl-4-methyl-1-piperazine Carboxamide Citrate
63. 1-piperazinecarboxamide, N,n-diethyl-4-methyl-, Citrate
64. Opera_id_558
65. 1-piperazinecarboxamide, N,n-diethyl-4-methyl-, Citrate (1:1)
66. N,n-diethyl-4-methyl-1-piperazinecarboxamide Dihydrogen Citrate (1:1)
67. Chembl937
68. 1-piperazinecarboxamide, N,n-diethyl-4-methyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
69. Schembl37736
70. Mls002207116
71. Mls004773909
72. Spectrum1500242
73. Chebi:4528
74. Dtxsid4045555
75. Hms501l10
76. Hms1568j10
77. Hms1920g06
78. Hms2091m16
79. Hms2095j10
80. Hms2234d17
81. Hms3370g20
82. Hms3712j10
83. Pharmakon1600-01500242
84. Tox21_110748
85. Ccg-40067
86. Nsc152050
87. Nsc756735
88. S4564
89. Akos003390253
90. Tox21_110748_1
91. Cs-3871
92. Diethylcarbamazine Citrate (jp17/usp)
93. Diethylcarbamazine Citrate [mi]
94. Nsc-152050
95. Nsc-756735
96. 2-hydroxypropane-1,2,3-tricarboxylic Acid; N,n-diethyl-4-methylpiperazine-1-carboxamide
97. Diethylcarbamazine Citrate [jan]
98. Diethylcarbamazine Citrate [hsdb]
99. Ncgc00017034-02
100. Ncgc00017034-03
101. Ncgc00090954-07
102. Ncgc00094651-01
103. Ncgc00094651-02
104. Bs-15595
105. Diethylcarbamazine Citrate [mart.]
106. Hy-12642
107. Diethylcarbamazine Citrate [usp-rs]
108. Diethylcarbamazine Citrate [who-dd]
109. Db-043586
110. D1898
111. Ft-0624487
112. C73172
113. D00803
114. Diethylcarbamazine Citrate [green Book]
115. Diethylcarbamazine Citrate [orange Book]
116. Diethylcarbamazine Citrate [usp Impurity]
117. A810546
118. Diethylcarbamazine Citrate [usp Monograph]
119. Wln: T6n Dntj Avn2&2 D1 &ov1xqvo&1vo
120. Sr-01000759234-2
121. Sr-01000759234-4
122. W-107945
123. Diethylcarbamazine Dihydrogen Citrate [who-ip]
124. Q27285812
125. Diethylcarbamazini Dihydrogenocitras [who-ip Latin]
126. 1-piperazinecarboxamide,n-diethyl-4-methyl-, Citrate (1:1)
127. Diethylcarbamazine Citrate Salt, Vetranal(tm), Analytical Standard
128. Diethylcarbamazine Citrate, British Pharmacopoeia (bp) Reference Standard
129. Diethylcarbamazine Citrate, European Pharmacopoeia (ep) Reference Standard
130. 1-piperazinecarboxamide,n-diethyl-4-methyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
131. Diethylcarbamazine Citrate, United States Pharmacopeia (usp) Reference Standard
132. N,n-diethyl-4-methyl-1-piperazinecarboxamide; 2-hydroxypropane-1,2,3-tricarboxylic Acid
133. 16354-46-4
| Molecular Weight | 391.42 g/mol |
|---|---|
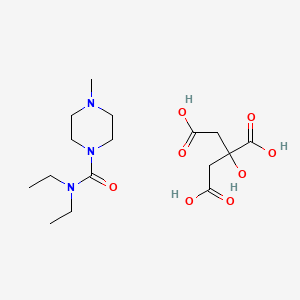
| Molecular Formula | C16H29N3O8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 391.19546489 g/mol |
| Monoisotopic Mass | 391.19546489 g/mol |
| Topological Polar Surface Area | 159 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Filaricides; Lipoxygenase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Dietylcarbamazine citrate is usually admin by mouth as tablets. ... It has also been given by intramuscular injection.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 91
For mass treatment /against microfilariae of Wucereria bancrofti and Wuchereria malayi/ with the objective of reducing microfilaremia to subinfective levels for mosquitoes, the dose is 2 mg/kg, three times daily after meals, for 7 days for treatment directed toward possible cure, this dosage regimen is carried out for 10 to 30 days. ... For practical purposes, an adequate amount seems to be a total dose of about 72 mg/kg of the citrate salt.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1010
For /treatment against Loa loa microfilariae/ a dose of 2 mg/kg should be given 3 times daily after meals for 2 to 3 weeks. If repeated courses are required to produce cure, they should be separated by periods of 3 to 4 weeks.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1011
For more Therapeutic Uses (Complete) data for DIETHYLCARBAMAZINE CITRATE (16 total), please visit the HSDB record page.
There are no contraindications to the use of diethylcarbamazine, other than the fact that low doses should be used for initial therapy, especially in onchocerciasis and infection due to Loa loa (to minimize adverse reactions to destruction of the parasites).
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1011
Special care should be taken in using diethylcarbamazine in areas where both onchocerciasis and loaiasis occur. /Diethylcarbamazine/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 91
In patients infected with Onchocerca volvulus or Wuchereria malayi, and to a lesser extent in those infected with Wuchereria bancrofti and Loa loa, the initial systemic reactions provoked by the massive destruction of microfilariae, or both during treatment may be severs. In such cases the dosage should be lowered or the drug stopped temporarily. Relief of these symptoms in heavily infected individuals may be afforded by pretreatment with corticosteroids, for example, dexamethasone (2 to 4 mg twice daily).
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1011
... use of diethylcarbamazine in microfilariae-positive dogs, is strictly contraindicated. ... The AVMA Council on Veterinary Services advises that all previously treated heartworm cases should be checked 3 months after the dog is started on diethylcarbamazine. If microfilariae are detected, the prophylactic program must be stopped until existing microfilariae are eliminated.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 849
For more Drug Warnings (Complete) data for DIETHYLCARBAMAZINE CITRATE (6 total), please visit the HSDB record page.
Filaricides
Pharmacological agents destructive to nematodes in the superfamily Filarioidea. (See all compounds classified as Filaricides.)
Lipoxygenase Inhibitors
Compounds that bind to and inhibit that enzymatic activity of LIPOXYGENASES. Included under this category are inhibitors that are specific for lipoxygenase subtypes and act to reduce the production of LEUKOTRIENES. (See all compounds classified as Lipoxygenase Inhibitors.)
Diethylcarbamazine is readily absorbed from the gastrointestinal tract. After a single oral dose of 200 to 400 mg, the concentration in plasma peaks in 1 to 2 hours ... Excretion is nearly all urinary, & more than 70% of the drug appears as metabolites. The compound is distributed almost completely throughout all body compartments with the exception of fat. Little accumulation occurs when repeated doses are given. /Diethylcarbamazine/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1010
Only 10-25% of the drug is excreted unchanged; the remainder is excreted as one of four known metabolites, all of which contain the intact piperazine ring.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 849
... the plasma half-life /in man/ is about 8 hr after a 200 mg dose and 12 hr after an 800 mg dose.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1010
The drug has two types of action on susceptible microfilariae. The first is to decrease the muscular activity and eventually immobilize the organisms; this may result from a hyerpolarizing effect of the piperazine moiety, and it causes dislocation of the parasites from their normal habitat in the host. The second action is to produce alterations in the microfilarial surface membranes, thereby rendering them more susceptible to destruction by host defense mechanisms. There is definite evidence that diethylcarbamazine kills adult worms of Loa loa and presumptive evidence that it kills adult Wuchereria bancrofti and Wuchereria malayi. However, it has little action against adult Onchocerca volvulus. The mechanism of the filaricidal action of diethylcarbamazine is unknown. /Diethylcarbamazine/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1010
It is postulated that in naturally infected animals, diethylcarbamazine somehow promotes the combination of antigen and antibody on the surface of serotonin-rich platelets. Serotonin is released from damaged platelets, which dramatically increases vascular permeability and leads to shock. This generally but not always occurs in dogs with a high microfilaremia.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 848
Diethylcarbamazine causes rapid disappearance of microfilariae of Wuchereriia bancrofti, Wuchereria malayi, and Loa loa from the blood of man. The drug causes microfilariae of Onchlcerca volvulus to disappear from the skin but does not kill microfilariae in nodules that contain the adult (female) worms. It does not affect the microfilariae of Wuchereria bancrofti in a hydrocele, despite penetration into the fluid.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1010