


1. Apo Diflunisal
2. Apo-diflunisal
3. Apodiflunisal
4. Dolobid
5. Dolobis
6. Dolocid
7. Mk 647
8. Mk-647
9. Mk647
10. Novo Diflunisal
11. Novo-diflunisal
12. Novodiflunisal
13. Nu Diflunisal
14. Nu-diflunisal
15. Nudiflunisal
1. 22494-42-4
2. Dolobid
3. Dolobis
4. Fluniget
5. Flovacil
6. Fluodonil
7. Adomal
8. Flustar
9. 5-(2,4-difluorophenyl)salicylic Acid
10. Mk-647
11. 5-(2,4-difluorophenyl)-2-hydroxybenzoic Acid
12. Diflusinal
13. Mk 647
14. 5-[2,4-difluorophenyl]salicylic Acid
15. 2',4'-difluoro-4-hydroxy-3-biphenylcarboxylic Acid
16. 2-(hydroxy)-5-(2,4-difluorophenyl)benzoic Acid
17. [1,1'-biphenyl]-3-carboxylic Acid, 2',4'-difluoro-4-hydroxy-
18. 2',4'-difluoro-4-hydroxybiphenyl-3-carboxylic Acid
19. 5-(2,4-difluorophenyl)-2-hydroxy-benzoic Acid
20. 2',4'-difluoro-4-hydroxy[1,1'-biphenyl]-3-carboxylic Acid
21. 2',4'-difluoro-4-hydroxy-(1,1'-biphenyl)-3-carboxylic Acid
22. Chembl898
23. Nsc-756728
24. (1,1'-biphenyl)-3-carboxylic Acid, 2',4'-difluoro-4-hydroxy-
25. Mls000028678
26. 7c546u4den
27. Algobid
28. Citidol
29. Difludol
30. Noaldol
31. Reuflos
32. 2',4'-difluoro-4-hydroxy-[1,1'-biphenyl]-3-carboxylic Acid
33. 3-biphenylcarboxylic Acid, 2',4'-difluoro-4-hydroxy-
34. Chebi:39669
35. Unisal
36. 2',4'-difluoro-4-hydroxy-biphenyl-3-carboxylic Acid
37. Ncgc00016765-01
38. Diflunisalum
39. Dolisal
40. Dolobil
41. Smr000058723
42. Cas-22494-42-4
43. Dsstox_cid_2932
44. Dsstox_rid_76792
45. Dsstox_gsid_22932
46. Diflunisalum [inn-latin]
47. 1fl
48. Dolobid (tn)
49. Mk647
50. Sr-01000003165
51. Einecs 245-034-9
52. Mfcd00057834
53. Brn 2654431
54. Unii-7c546u4den
55. Diflunisal (jan/usp/inn)
56. Prestwick_168
57. Diflunisal [usan:usp:inn:ban:jan]
58. Spectrum_000962
59. Diflunisal [mi]
60. Opera_id_803
61. Diflunisal [inn]
62. Diflunisal [jan]
63. Prestwick0_000039
64. Prestwick1_000039
65. Prestwick2_000039
66. Prestwick3_000039
67. Spectrum2_001012
68. Spectrum3_000392
69. Spectrum4_000513
70. Spectrum5_000901
71. Diflunisal [usan]
72. Diflunisal [vandf]
73. Diflunisal [mart.]
74. Schembl4337
75. Diflunisal [usp-rs]
76. Diflunisal [who-dd]
77. Bspbio_000137
78. Bspbio_002203
79. Kbiogr_001085
80. Kbioss_001442
81. Mls001146895
82. Bidd:gt0063
83. Divk1c_000938
84. Spectrum1500245
85. Spbio_001163
86. Spbio_002058
87. Diflunisal, Analytical Standard
88. Bpbio1_000151
89. Gtpl7162
90. Diflunisal [ep Impurity]
91. Diflunisal [orange Book]
92. Dtxsid5022932
93. Hms502o20
94. Kbio1_000938
95. Kbio2_001442
96. Kbio2_004010
97. Kbio2_006578
98. Kbio3_001423
99. Zinc20243
100. Diflunisal [usp Impurity]
101. Ninds_000938
102. Diflunisal [usp Monograph]
103. Hms1568g19
104. Hms1920g10
105. Hms2090c16
106. Hms2091m20
107. Hms2095g19
108. Hms3259g17
109. Hms3712g19
110. Hms3885h10
111. Pharmakon1600-01500245
112. Bcp09905
113. Tox21_110598
114. Bdbm50240510
115. Ccg-40230
116. Nsc756728
117. S4609
118. Akos005762917
119. Tox21_110598_1
120. Db00861
121. Ks-1346
122. Nc00506
123. Nsc 756728
124. Idi1_000938
125. Ncgc00016765-02
126. Ncgc00016765-03
127. Ncgc00016765-04
128. Ncgc00016765-05
129. Ncgc00016765-06
130. Ncgc00016765-08
131. Ncgc00016765-09
132. Ncgc00022783-03
133. Ncgc00022783-04
134. 1286107-99-0
135. Diflunisal 100 Microg/ml In Acetonitrile
136. Hy-18342
137. Sbi-0051347.p003
138. Db-045934
139. Ab00051969
140. Cs-0007468
141. D5875
142. Ft-0630487
143. C01691
144. D00130
145. Ab00051969-12
146. Ab00051969_13
147. A816230
148. 2',4'-difluoro-4-hydroxybipheny-3-carboxylic Acid
149. J-014739
150. Q2602750
151. Sr-01000003165-2
152. Sr-01000003165-3
153. Brd-k22031190-001-05-3
154. Brd-k22031190-001-13-7
155. 5-(2,4-difluorophenyl)-2-hydroxy-benzoic Acid;diflunisal
156. Diflunisal, European Pharmacopoeia (ep) Reference Standard
157. 2',4'-difluoro-4-hydroxy-(1',1-diphenyl)-3-carboxylic Acid
158. Diflunisal, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 250.20 g/mol |
|---|---|
| Molecular Formula | C13H8F2O3 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 250.04415044 g/mol |
| Monoisotopic Mass | 250.04415044 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 311 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Diflunisal |
| PubMed Health | Diflunisal (By mouth) |
| Drug Classes | Analgesic, Antirheumatic |
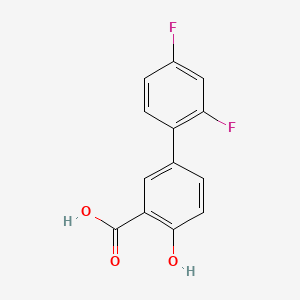
| Drug Label | Diflunisal is [1, 1-Biphenyl]-3-carboxylic acid, 2, 4-difluoro-4-hydroxy. Its structural formula is:Molecular Formula: C13H8F2O3Molecular Weight: 250.20 g/molDiflunisal is a stable, white, crystalline compound with a melting point of 211... |
| Active Ingredient | Diflunisal |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Teva; Emcure Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Diflunisal |
| PubMed Health | Diflunisal (By mouth) |
| Drug Classes | Analgesic, Antirheumatic |
| Drug Label | Diflunisal is [1, 1-Biphenyl]-3-carboxylic acid, 2, 4-difluoro-4-hydroxy. Its structural formula is:Molecular Formula: C13H8F2O3Molecular Weight: 250.20 g/molDiflunisal is a stable, white, crystalline compound with a melting point of 211... |
| Active Ingredient | Diflunisal |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Teva; Emcure Pharms Usa |
For symptomatic treatment of mild to moderate pain accompanied by inflammation (e.g. musculoskeletal trauma, post-dental extraction, post-episiotomy), osteoarthritis, and rheumatoid arthritis.
FDA Label
Diflunisal is a nonsteroidal drug with analgesic, anti-inflammatory and antipyretic properties. It is a peripherally-acting non-narcotic analgesic drug. Habituation, tolerance and addiction have not been reported. Diflunisal is a difluorophenyl derivative of salicylic acid. Chemically, diflunisal differs from aspirin (acetylsalicylic acid) in two respects. The first of these two is the presence of a difluorophenyl substituent at carbon 1. The second difference is the removal of the 0-acetyl group from the carbon 4 position. Diflunisal is not metabolized to salicylic acid, and the fluorine atoms are not displaced from the difluorophenyl ring structure.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA11 - Diflunisal
Absorption
Rapidly and completely absorbed following oral administration, with a bioavailability of 80-90%. Peak plasma concentrations are achieved 2 - 3 hours following oral administration.
Route of Elimination
The drug is excreted in the urine as two soluble glucuronide conjugates accounting for about 90% of the administered dose. Little or no diflunisal is excreted in the feces.
Hepatic, primarily via glucuronide conjugation (90% of administered dose).
8 to 12 hours
The precise mechanism of the analgesic and anti-inflammatory actions of diflunisal is not known. Diflunisal is a prostaglandin synthetase inhibitor. In animals, prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain. Since prostaglandins are known to be among the mediators of pain and inflammation, the mode of action of diflunisal may be due to a decrease of prostaglandins in peripheral tissues.