


1. C.m.9155
2. Env905
3. Epitopic
1. 23674-86-4
2. Durezol
3. Dfba
4. Myser
5. Epitopic
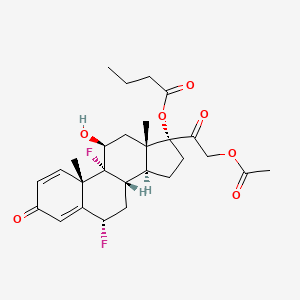
6. [(6s,8s,9r,10s,11s,13s,14s,17r)-17-(2-acetyloxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Butanoate
7. Difluoroprednisolone Butyrate Acetate
8. W 6309
9. Cm 9155
10. S8a06qg2qe
11. Difluprednatum
12. Mls000028663
13. Mls001148580
14. Chebi:31485
15. W-6309
16. Smr000058924
17. (6alpha,11beta)-21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)pregna-1,4-diene-3,20-dion
18. Difluprednato
19. 6alpha,9alpha-difluoroprednisolone 21-acetate 17-butyrate
20. Durezol (tn)
21. Unii-s8a06qg2qe
22. Difluprednatum [inn-latin]
23. Difluprednato [inn-spanish]
24. Difluprednate [usan:inn:jan]
25. Ncgc00168749-01
26. Einecs 245-815-4
27. Mfcd00214273
28. Opera_id_1287
29. Difluprednate [mi]
30. Difluprednate [inn]
31. Difluprednate [jan]
32. Schembl4580
33. Difluprednate [usan]
34. Dsstox_cid_26773
35. Dsstox_rid_81894
36. Dsstox_gsid_46773
37. Difluprednate [vandf]
38. Mls001333701
39. St60-1
40. Difluprednate [mart.]
41. Difluprednate [who-dd]
42. Gtpl7474
43. Chembl1201749
44. Difluprednate (jan/usan/inn)
45. Dtxsid0046773
46. 6-alpha,9-alpha-difluoroprednisolone 17-butyrate 21-acetate
47. Hms2231d18
48. Difluprednate [orange Book]
49. (6s,8s,9r,10s,11s,13s,14s,17r)-17-(2-acetoxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl Butyrate
50. Act03289
51. Zinc4212945
52. Tox21_112628
53. S4095
54. Ccg-269762
55. Db06781
56. 6alpha,9-difluoro-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate
57. 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)pregna-1,4-diene-3,20-dione (6alpha,11beta)-
58. As-15800
59. Hy-17569
60. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6.alpha.,11.beta.)-
61. Cas-23674-86-4
62. D5579
63. D01266
64. Ab00383058_10
65. 674d864
66. A932746
67. Q736113
68. Sr-01000000265
69. Q-101389
70. Sr-01000000265-4
71. 6alpha-9-difluoroprednisolone 21-acetate 17-butyrate
72. 6 Alpha ,9 Alpha -difluoroprednisolone 21-acetate 17-butyrate
73. 6alpha,9alpha-difluoroprednisolone 21-acetate 17-butyrate, >=98%
74. (1r,2s,8s,10s,11s,14r,15s,17s)-14-[2-(acetyloxy)acetyl]-1,8-difluoro-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl Butanoate
75. 6.alpha.,9-difluoro-11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate
76. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6alpha,11beta)-
77. Pregna-1,4-diene-3,20-dione, 6-alpha,9-difluoro-11-beta,17,21-trihydroxy-, 21-acetate, 17-butyrate
78. Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6a,11b)-
| Molecular Weight | 508.5 g/mol |
|---|---|
| Molecular Formula | C27H34F2O7 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 508.22725974 g/mol |
| Monoisotopic Mass | 508.22725974 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Durezol |
| PubMed Health | Difluprednate (Into the eye) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Durezol (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6,9difluoro-11,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-... |
| Active Ingredient | Difluprednate |
| Dosage Form | Emulsion |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Durezol |
| PubMed Health | Difluprednate (Into the eye) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Durezol (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6,9difluoro-11,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-... |
| Active Ingredient | Difluprednate |
| Dosage Form | Emulsion |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon Pharms |
For the treatment of inflammation and pain associated with ocular surgery.
FDA Label
Difluprednate is a corticosteroid used as an anti-inflammatory steroidal drug used primarily in ocular surgery.
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC19 - Difluprednate
Absorption
Difluprednate penetrates the corneal epithelium rapidly and effectively. Low systemic absorption.
Route of Elimination
78.5% of radioactivity was excreted aftert 24 hours, and 99.5% by 7 days after a single dose of labeled difluprednate instilled in the right eyes of pigmented rabbits.
Difluprednate is rapidly deacetylated in the aqueous humor to difluoroprednisolone butyrate (DFB), the drugs active metabolite. Endogenous tissue esterases then metabolize DFB to the inert metabolite hydroxyfluoroprednisolone butyrate (HFB), which limits systemic exposure to the active compound.
Corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins (lipocortins). It is postulated that these proteins control the biosynthesis of potent mediators of infammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
