
1. Alpha Artemether
2. Alpha-artemether
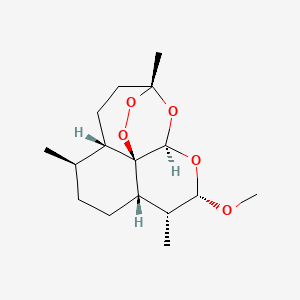
3. Artemether, (3r-(3alpha,5abeta,6alpha,8abeta,9alpha,10beta,12beta,12ar*))-isomer
4. Artemether, (3r-(3alpha,5abeta,6beta,8aalpha,9alpha,10beta,12beta,12ar*))-isomer
5. Artemether, (3r-(3alpha,5abeta,6beta,8abeta,9alpha,10alpha,12beta,12ar*))-isomer
6. Artenam
7. Beta Arthemeter
8. Beta-arthemeter
9. O Methyldihydroartemisinine
10. O-methyldihydroartemisinine
1. 71963-77-4
2. Beta-artemether
3. Dihydroartemisinin Methyl Ether
4. Artemetherum
5. Artemisininelactol Methyl Ether
6. Methyl-dihydroartemisinine
7. Artemetero
8. Paluther
9. Beta-dihydroartemisinin Methyl Ether
10. Sm 224
11. Artemetheri
12. Dihydroqinghaosu Methyl Ether
13. Artesaph
14. Falcidol
15. Gvither
16. Malartem
17. Chebi:195280
18. Larither
19. Artenam
20. Artemos
21. Cpd000469218
22. (1r,4s,5r,8s,9r,10s,12r,13r)-10-methoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0^{4,13}.0^{8,13}]hexadecane
23. .beta.-artemether
24. Sm-224
25. Artemetero [inn-spanish]
26. Artemetherum [inn-latin]
27. Methoxy(trimethyl)[?]
28. 10-methoxy-1,5,9-trimethyl-(1r,4s,5r,8s,9r,10s,12r,13r)-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecane
29. Dihydroquinghaosu Methyl Ether
30. Hsdb 7456
31. C7d6t3h22j
32. Nsc 665970
33. Unii-c7d6t3h22j
34. Artemeter
35. Artimist
36. Qinghao
37. Beta Artemether
38. Artemether [usan:inn:ban]
39. Cgp 56696
40. Nsc665970
41. Nsc-665970
42. Nsc-759820
43. (1r,4s,5r,8s,9r,10s,12r,13r)-10-methoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecane
44. (3r,5as,6r,8as,9r,10s,12r,12ar)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin
45. (3r,5as,6r,8as,9r,10s,12r,12ar)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12h-pyrano[4,3-j]-1,2-benzodioxepin
46. Dihydroartemisinin Impurity G
47. Mls001424249
48. Artemether (jan/usan/inn)
49. Chembl566534
50. Gtpl9955
51. Schembl1650501
52. Artemether, >=98% (hplc)
53. Dtxsid7040651
54. Hms2052l09
55. Hms2232j21
56. Sm224
57. 3,12-epoxy-12h-pyrano[4,3-j]-1,2-benzodioxepin, Decahydro-10-methoxy-3,6,9-trimethyl-, (3r,5as,6r,8as,9r,10s,12r,12ar)-
58. Act03313
59. Amy25769
60. Hy-n0402
61. Bdbm50248200
62. Mfcd00866205
63. Zinc14263142
64. Akos026750084
65. Ccg-101180
66. Db06697
67. Nc00430
68. 3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin, Decahydro-10-methoxy-3,6,9-trimethyl-, (3-alpha,5a-beta,6-beta,8a-beta,9-alpha,12-beta,12ar)-, (+)-
69. Smr000469218
70. D02483
71. Ab00698368-05
72. Ab00698368_06
73. Q416199
74. 2-chloro-3,5-dimethyl-4-methoxypyridinehydrochloride
75. Z1550675461
76. Artemether, United States Pharmacopeia (usp) Reference Standard
77. (1r,4s,5r,8s,9r,10s,12r,13r)-10-methoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0;{4,13}.0;{8,13}]hexadecane
78. (3r,5as,6r,8as,9r,10s,12r,12ar)-10-methoxy-3,6,9-trimethyldecahydro-3,12-epoxypyrano[4,3-j][1,2]benzodioxepine
79. (3r,5as,6r,8as,9r,10s,12r,12ar)-10-methoxy-3,6,9-trimethyldecahydro-3h-3,12-epoxy[1,2]dioxepino[4,3-i]isochromene
80. (3r,5as,6r,8as,9r,10s,12r,12ar)-decahydro-10methoxy-3,6,9-trimethyl-3,12-epoxy-12h-pyrano[4,3-j]-1,2benzodioxepin
81. D8z
| Molecular Weight | 298.37 g/mol |
|---|---|
| Molecular Formula | C16H26O5 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 298.17802393 g/mol |
| Monoisotopic Mass | 298.17802393 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 429 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | COARTEM |
| Active Ingredient | ARTEMETHER; LUMEFANTRINE |
| Company | NOVARTIS (Application Number: N022268) |
MESH Heading: Antimalarial, antifungal, antiprotozoal, coccidiostats, schistosomicides
National Library of Medicine, SIS; ChemIDplus Record for Artemether (71963-77-4), MESH Heading. Available from, as of July 26, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Therap Cat: Antimalarial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 140
To counter the threat of resistance of P. falciparum to monotherapies, and to improve treatment outcome, combinations of antimalarials are now recommended by WHO for the treatment of falciparum malaria. ...The following ACTs are currently recommended: artemether-lumefantrine.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
Artemether-lumefantrine: This is currently available as co-formulated tablets .... The total recommended treatment is a 6-dose regimen of artemether-lumefantrine twice a day for 3 days. An advantage of this combination is that lumefantrine is not available as a monotherapy and has never been used by itself for the treatment of malaria. Recent evidence indicates that the therapeutic response and safety profile in young children of less than 10 kg is similar to that in older children, and artemether-lumefantrine is now recommended for patients 5 kg. Lumefantrine absorption is enhanced by co-administration with fat. Low blood levels, with resultant treatment failure, could potentially result from inadequate fat intake, and so it is essential that patients or carers are informed of the need to take this ACT /antimalarial combination therapy/ with milk or fat-containing food -- particularly on the second and third days of treatment.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
For more Therapeutic Uses (Complete) data for ARTEMETHER (11 total), please visit the HSDB record page.
Transient first-degree heart block, dose-related reversible decreases in reticulocyte and neutrophil counts, and temporary elevations of serum aspartate aminotransferase activity have been reported ... Brief episodes of drug-induced fever in human volunteers were noted in some studies ... /Artemisinin drugs/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1075
Because high doses of artemisinin drugs can produce neurotoxicity, prolongation of the QT interval, bone marrow depression, and fetal reabsorption in experimental animals, the possibility of long-term toxicity in human beings exists. /Artemisinin drugs/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1075
Some patients cannot tolerate oral treatment, and will require parenteral or rectal administration for 1-2 days until they can swallow and retain oral medication reliably. Although such patients may not show signs of severity, they should receive the same antimalarial dose regimens as for severe malaria.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
Some patients may have no signs of severity but on examination of the blood film are found to have very high parasitaemia. The risks associated with high parasitaemia vary depending on the age of the patient and on transmission intensity. Thus cut-off values and definitions of hyperparasitaemia also vary. Patients with high parasitaemias are at an increased risk of treatment failure and of developing severe malaria, and therefore have an increased risk of dying. These patients can be treated with the oral Antimalarial Combination Therapies (ACTs) recommended for uncomplicated malaria. However, they require close monitoring to ensure that the drugs are retained and that signs of severity do not develop, and they may require a longer course of treatment to ensure cure.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
For more Drug Warnings (Complete) data for ARTEMETHER (18 total), please visit the HSDB record page.
Artemether and lumefantrine combination therapy is indicated for the treatment of acute uncomplicated malaria caused by Plasmodium falciparum, including malaria acquired in chloroquine-resistant areas. May also be used to treat uncomplicated malaria when the Plasmodium species has not been identified. Indicated for use in adults and children greater than 5 kg.
FDA Label
In the body, artemether is metabolized into the active metabolite metabolite dihydroartemisinin. The drug works against the erythrocytic stages of P. falciparum by inhibiting nucleic acid and protein synthesis. Artemether is administered in combination with lumefantrine for improved efficacy. Artemether has a rapid onset of action and is rapidly cleared from the body. It is thought that artemether provides rapid symptomatic relief by reducing the number of malarial parasites. Lumefantrine has a much longer half life and is believed to clear residual parasites.
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01B - Antimalarials
P01BE - Artemisinin and derivatives, plain
P01BE02 - Artemether
Absorption
Food increases absorption.
Little or none of the administered drugs or dihydroartemisinin is recovered in urine. /Dihydroartemisinin/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1072
After intramuscular administration pharmacokinetics indicated peak plasma levels of artemether (AM) at 2 to 4 hours post-dose, slow elimination and a tendency to accumulate after repeated administration. Only low levels of the major metabolite, dihydroartemisinin (DHA), were found. AM levels in the cerebrospinal fluid (CSF) were < 10% of plasma levels. After oral administration AM concentrations were considerably lower than after i.m. administration. The concentration of DHA was high on day 1 but almost nil on day 7 indicating its fast inactivation in dogs. Two hours after the 8th oral administration neither AM nor DHA was detected in CSF which may explain the absence of neurotoxicity in dogs after oral administration of AM.
PMID:10661809 Classen W et al; Exp Toxicol Pathol 51 (6): 507-16 (1999)
The pharmacokinetics of intramuscular artemether and its major plasma metabolite-dihydroartemisinin, were investigated in patients with severe manifestations of falciparum malaria. Six severe falciparum malaria patients with acute renal failure (ARF) and 11 without ARF were recruited into the study. They were treated with intramuscular artemether at a loading dose of 160 mg, followed by daily doses of 80 mg for another 6 days (total dose 640 mg). Patients with and without ARF showed a good initial response to treatment; the parasite and fever clearance times were 66 (30 to 164) and 76 (36 to 140) hr (median (range)), respectively. None had reappearance of parasitaemia in their peripheral blood smear within 7 days of initiation of treatment. In comatose patients, the time to recovery of consciousness was 51.6 (22 to 144) hr. Artemether was detected in plasma as early as 1hr after a 160 mg dose, and declined to undetectable levels within 24 hr in most cases. Patients with ARF had significantly higher Cmax (2.38 (1.89 to 3.95) vs 1.56 (1.05 to 3.38) ng/mL/mg dose), and lower Vz/F (5.45 (3.2 to 6.9) vs 8.6 (4.2 to 12.3) L/kg) and CL/F (7.4 (5.4 to 13.8) vs 19.1 (8.5 to 25.1) mL/min/kg) when compared to those without ARF. In addition, t1/2z, was significantly longer in ARF patients (7.0 (5.5 to 10.0) vs 5.7 (4.2 to 6.6) hr). The parmacokinetics of dihydroartemisinin in the two groups were comparable. ARF significantly modified the pharmacokinetics of intramuscular artemether. The changes could be contributed to either improved absorption/bioavailability, a reduction of systemic clearance, or a change in plasma protein binding of the drug.
PMID:9663816 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1873641 Karbwang J et al; Br J Clin Pharmacol 45: 597-600 (1998)
Dihydroartemisinin is rapidly absorbed following oral administration, reaching peak levels after around 2.5 hr. Absorption via the rectal route is somewhat slower, with peak levels occurring around 4 hr after administration. Plasma protein binding is around 55%. Elimination half-life is approximately 45 min via intestinal and hepatic glucuronidation. /Dihydroartemisinin/
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
For more Absorption, Distribution and Excretion (Complete) data for ARTEMETHER (6 total), please visit the HSDB record page.
Rapidly metablized to its active metabolite, dihydroartemisinin.
Artemether ... /is/ converted to dihydroartemisinin ... The antimalarial effect of artemisinin compounds results primarily from dihydroartemisinin ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1072
Artemisinin is completely and rapidly absorbed after oral administration in rats. However, a very low plasma level was obtained even after a dose of 300 mg/kg. Liver was found to be the chief site of inactivation. When artemisinin was given i.m., significant and more persistent plasma levels were detected. Artemisinin was shown to pass the blood-brain and blood-placenta barriers after i.v. injection. Very little unchanged artemisinin was found in the urine or feces in 48 hours regardless of the route of administration. Metabolites identified after administration to humans include deoxyartemisinin, deoxydihydroartemisinin, and 9,10-dihydroxydeoxyartemisinin. /Artemisinin/
PMID:2084705 Lee IS, Hufford CD; Pharmacol Ther. 48 (3): 345-355 (1990)
Artemether, 1.6 +/- 0.7 and 2.2 +/- 1.9 hr; Dihydroartemisinin, 1.6 +/- 0.6 and 2.2 +/- 1.5 hr
Artemether ... /is/ converted to dihydroartemisinin ... which rapidly disappears from plasma with a half-life of about 45 min.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1072
Involves an interaction with ferriprotoporphyrin IX (heme), or ferrous ions, in the acidic parasite food vacuole, which results in the generation of cytotoxic radical species. The generally accepted mechanism of action of peroxide antimalarials involves interaction of the peroxide-containing drug with heme, a hemoglobin degradation byproduct, derived from proteolysis of hemoglobin. This interaction is believed to result in the formation of a range of potentially toxic oxygen and carbon-centered radicals.
Artemether (AM) is an antimalarial drug derived from artemisinin (Qinghaosu), an extract of the herb Artemisia annua L., sweet wormwood. Its antiparasitic effect is that of a schizontocide and is explained by rapid uptake by parasitized erythrocytes and interaction with a component of hemoglobin degradation resulting in formation of free radicals. It has been shown to exhibit a high clinical cure rate.
PMID:10661809 Classen W et al; Exp Toxicol Pathol 51 (6): 507-16 (1999)
Two theories have been put forward for the mode of antimalarial action of the artemisinin antimalarials, in accodance with the known properties of peroxides with medicinal activity. The first assumes that the artemisinins must be activated by contact with either reduced haem (ferrous haem, Fe(ll)PPIX) or non-haem ferrous iron (exogenous iron), causing cleavage of the peroxide to generate oxygen-centered radicals (alkoxy radicals') which are then presumed to be converted into carbon-centered radicals by transfer of proximate hydrogen atoms from the periphery of the peroxide molecule. These carbon-centered radicals are then thought to alkylate sensitive, yet unspecified, biomolecules in the parasite. A second theory argues for a process in which the intact artemisinin binds to a site within a vital protein in the parasite. The act of binding causes the peroxide to be converted to hydroperoxide or similar open peroxide, which in accordance with known properties of such compounds, generates one or more active chemical entities, either oxidizing agents or oxygen transfer agents per se, or oxygen-centered free radicals. This would be associated with the binding process. In such a way, the artemisinins might act as (irreversibile) inhibitors. Iron may, or may not, be associated with the activation process. No specific biological target in the parasite has yet been identified in support of this theory, but it may be membrane-bound proteins.
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp