
1. 00012, Bg
2. 12 Compound, Bg
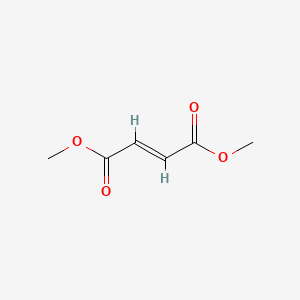
3. 2-butenedioic Acid, (2e)-, Dimethyl Ester
4. 201, Fag
5. Bg 00012
6. Bg 12 Compound
7. Bg-00012
8. Bg-12 Compound
9. Bg00012
10. Bg12 Compound
11. Compound, Bg 12
12. Compound, Bg12
13. Dimethylfumarate
14. Fag 201
15. Fag-201
16. Fag201
17. Fumaderm
18. Fumarate, Dimethyl
19. Tecfidera
1. 624-49-7
2. Tecfidera
3. Dimethylfumarate
4. Methyl Fumarate
5. (e)-dimethyl Fumarate
6. Fumaderm
7. Dimethyl (e)-but-2-enedioate
8. Fumaric Acid Dimethyl Ester
9. Fumaric Acid, Dimethyl Ester
10. Bg-12
11. Boletic Acid Dimethyl Ester
12. Dimethyl Trans-ethylenedicarboxylate
13. Bg 12 Compound
14. Trans-butenedioic Acid Dimethyl Ester
15. Panaclar
16. Bg00012
17. 2-butenedioic Acid (e)-, Dimethyl Ester
18. Allomaleic Acid Dimethyl Ester
19. Bg-00012
20. Dimethyl 2-butenedioate
21. Trans-1,2-ethylenedicarboxylic Acid Dimethyl Ester
22. Dimethyl (2e)-but-2-enedioate
23. Fag-201
24. Bg 00012
25. Bis-methyl Ester
26. Dimethyl Fumar
27. Dimethyl Fumarate [usan]
28. Azl O 211089
29. Azl-o-211089
30. Nsc-25942
31. Dimethylester Kyseliny Fumarove
32. Nsc-167432
33. (e)-but-2-enedioic Acid Dimethyl Ester
34. Chebi:76004
35. 2-butenedioic Acid (2e)-, Dimethyl Ester
36. Bg 12
37. Fp187
38. Las41008
39. Fp-187
40. Las-41008
41. Fumaric Acid-dimethyl Ester
42. Azl-0211089
43. 1,2-bis(methoxycarbonyl)-trans-ethylene
44. Dimethyl (2e)-2-butenedioate
45. Fo2303mni2
46. 23055-10-9
47. Dimethyl (~{e})-but-2-enedioate
48. But-2-enedioic Acid, Dimethyl Ester
49. Azl 0 211089
50. Dimethyl Fumarate (usan)
51. 2-butenedioic Acid (2e)-, 1,4-dimethyl Ester
52. Wln: 1ov1u1vo1 -t
53. Fumaric Acid-dimethyl Ester 1000 Microg/ml In Acetonitrile
54. 2-butenedioic Acid, Dimethyl Ester
55. Mfcd00064438
56. Ethylene,2-bis(methoxycarbonyl)-, Trans-
57. Fag201
58. Fag 201
59. Bg 12 [fumarate]
60. Bg-12 [fumarate]
61. Einecs 210-849-0
62. Nsc 25942
63. Tl 353
64. Nsc 167432
65. Dimethylester Kyseliny Fumarove [czech]
66. Brn 0774590
67. Unii-fo2303mni2
68. Ai3-07872
69. Dimethyl-fumarate
70. Fumaric Acid Dimethyl Ester (1,1,1,8,8,8-d6)
71. Ethylene, 1,2-bis(methoxycarbonyl)-, Trans-
72. Hsdb 7725
73. Tecfidera (tn)
74. Fumaric Acid Dimethyl
75. 2-butenedioic Acid, (2e)-, Dimethyl Ester
76. Dimethyl Fumarate, 97%
77. (e/z)-dimethyl Fumarate
78. Dimethyl Trans-butenedioate
79. Schembl41835
80. Schembl41836
81. 4-02-00-02205 (beilstein Handbook Reference)
82. Dimethyl Fumarate [mi]
83. Dimethyl Fumarate (jan/usan)
84. Gtpl7045
85. Dimethyl Fumarate [jan]
86. Chembl2107333
87. Dimethyl Fumarate [hsdb]
88. Dtxsid4060787
89. Dimethyl Fumarate [vandf]
90. But-2-enedioic Aciddimethyl Ester
91. Hms3264d14
92. Pharmakon1600-01506154
93. Dimethyl Fumarate [who-dd]
94. Nsc25942
95. Zinc3843378
96. Bdbm50504654
97. Fumaric Acid, Dimethyl Ester (8ci)
98. Nsc167432
99. Nsc760139
100. S2586
101. Stk039379
102. Dimethyl Ester(e)-2-butenedioic Acid
103. (e)-ch3oc(o)ch=chc(o)och3
104. Akos000121333
105. Zinc100509880
106. Ccg-213618
107. Cs-0909
108. Db08908
109. Dimethyl Ester(2e)-2-butenedioic Acid
110. Dimethyl Fumarate [orange Book]
111. Nsc-760139
112. Hy-17363
113. Ls-13141
114. 2(e)-butenedioic Acid 1,4-dimethyl Ester
115. 2-butenedioic Acid, Dimethyl Ester, (2e)-
116. Cs-0369103
117. F0069
118. Sw219154-1
119. D03846
120. H11241
121. Ab00172980_03
122. Ab00172980_04
123. Dimethyl Fumarate, Vetec(tm) Reagent Grade, 97%
124. Q418123
125. Sr-01000944222
126. Sr-01000944222-1
127. Trans-1, 2-ethylenedicarboxylic Acid Dimethyl Ester
128. Brd-k31111078-001-01-8
129. Fumaric Acid Dimethyl Ester 100 Microg/ml In Methanol
130. F0001-1675
131. Dimethyl Fumarate, Certified Reference Material, Tracecert(r)
132. 12287-98-8
133. Eou
| Molecular Weight | 144.12 g/mol |
|---|---|
| Molecular Formula | C6H8O4 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 144.04225873 g/mol |
| Monoisotopic Mass | 144.04225873 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 141 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Tecfidera |
| PubMed Health | Dimethyl Fumarate (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | TECFIDERA contains dimethyl fumarate which is also known by its chemical name, dimethyl (E) butenedioate, (C6H8O4). It has the following structure: Dimethyl fumarate is a white to off-white powder that is highly soluble in water with a molecular mass... |
| Active Ingredient | Dimethyl fumarate |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | 120mg; 240mg |
| Market Status | Prescription |
| Company | Biogen Idec |
| 2 of 2 | |
|---|---|
| Drug Name | Tecfidera |
| PubMed Health | Dimethyl Fumarate (By mouth) |
| Drug Classes | Immune Modulator |
| Drug Label | TECFIDERA contains dimethyl fumarate which is also known by its chemical name, dimethyl (E) butenedioate, (C6H8O4). It has the following structure: Dimethyl fumarate is a white to off-white powder that is highly soluble in water with a molecular mass... |
| Active Ingredient | Dimethyl fumarate |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | 120mg; 240mg |
| Market Status | Prescription |
| Company | Biogen Idec |
Dermatologic Agents; Immunosuppressive Agents; Radiation-Sensitizing Agents
National Library of Medicine's Medical Subject Headings. Dimethyl Fumarate. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Dimethyl fumarate is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=Dimethyl+Fumarate
Tecfidera is indicated for the treatment of patients with relapsing forms of multiple sclerosis. /Included in US product label/
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
EXPL THER Mixtures of fumaric acid esters (FAE) are used as an oral systemic treatment for moderate to severe psoriasis. Large clinical studies with dimethylfumarate (DMF) monotherapy are scarce. The objective of this study is to assess the effectiveness and long-term safety of high-dose DMF monotherapy in moderate to severe psoriasis. A prospective single-blinded follow-up study was performed in a cohort of patients treated with DMF. Patients were followed-up at fixed intervals. Assessment of consecutive photographs was performed by two observers. Primary outcome was a change in static physician global assessment (PGA) score. Safety outcome was defined as incidences of (serious) adverse events. A total of 176 patients with moderate to severe psoriasis were treated with DMF for a median duration of 28 months. The median daily maintenance dosage of 480 mg was reached after a median of 8 months. Psoriasis activity decreased significantly by 1.7 out of five points. A total of 152 patients reported one or more adverse events, such as gastrointestinal complaints and flushing. High-dose DMF monotherapy is an effective and safe treatment option in moderate to severe psoriasis. It can be suggested that 50% of all patients may benefit from high-dose DMF monotherapy. KEYWORDS: Dimethylfumurate; high dose; monotherapy; prospective study; psoriasis
PMID:26088405 Lijnen R et al; J Dermatolog Treat. 2015 Jun 19:1-6. (Epub ahead of print)
A patient with multiple sclerosis who was being treated with dimethyl fumarate developed progressive multifocal leukoencephalopathy (PML), and later died. The patient who died was not taking any other drugs that affect the immune system or drugs that are thought to be associated with PML. Patients taking dimethyl fumarate should be advised to contact their clinician if they develop any symptoms that may be suggestive of PML. Symptoms of PML are diverse, progress over days to weeks, and include the following: progressive weakness on one side of the body or clumsiness of limbs; disturbance of vision; and changes in thinking, memory and orientation, leading to confusion and personality changes. The progression of deficits can lead to severe disability or death. Dimethyl fumarate should be discontinued immediately at the first sign or symptom suggestive of PML and an appropriate diagnostic evaluation should be performed. Lymphocyte counts should be monitored in dimethyl fumarate-treated patients according to approved labeling.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3620
Dimethyl fumarate may decrease lymphocyte counts. In placebo-controlled clinical trials, mean lymphocyte counts decreased by approximately 30% during the first year of treatment with the drug and remained stable thereafter. Mean lymphocyte counts improved 4 weeks following discontinuance of the drug, but did not return to baseline values. Dimethyl fumarate has not been studied in patients with preexisting low lymphocyte counts. Prior to initiation of dimethyl fumarate, a recent (i.e., within 6 months) complete blood cell (CBC) count should be available to identify patients with preexisting low lymphocyte counts. A CBC should also be obtained annually during therapy and as clinically indicated. In patients with serious infections, withholding dimethyl fumarate treatment should be considered until the infection has resolved.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3620
During post marketing experience, hypersensitivity reactions have been reported, including rare reports of anaphylaxis and angioedema in patients treated with Tecfidera. Signs and symptoms have included difficulty breathing, urticaria, and swelling of the throat and tongue.
Health Canada; Product Monograph for Tecfidera (Dimethyl Fumarate) Delayed-release Capsules, Drug Identification Number (DIN): 02404508 p.14 (Date of Revision: January 29, 2015). Available from, as of June 30, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
Treatment with Tecfidera should not be initiated in patients with signs and symptoms of a serious infection. Decreases in lymphocyte counts observed in patients treated with Tecfidera in clinical trials were not associated with increased frequencies of infections. However, due to the potential risk of infections in patients who develop sustained lymphopenia, patients should be instructed to report symptoms of infection to their physician. For patients with signs and symptoms of serious infections, interrupting treatment with Tecfidera should be considered, until the infection(s) resolves.
Health Canada; Product Monograph for Tecfidera (Dimethyl Fumarate) Delayed-release Capsules, Drug Identification Number (DIN): 02404508 p.6 (Date of Revision: January 29, 2015). Available from, as of June 30, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
For more Drug Warnings (Complete) data for DIMETHYL FUMARATE (14 total), please visit the HSDB record page.
Used in multiple sclerosis patients with relapsing forms.
FDA Label
Tecfidera is indicated for the treatment of adult and paediatric patients aged 13 years and older with relapsing remitting multiple sclerosis (RRMS).
Skilarence is indicated for the treatment of moderate to severe plaque psoriasis in adults in need of systemic medicinal therapy.
Treatment of psoriasis
Treatment of multiple sclerosis
Dimethyl fumarate Mylan is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis.
Dimethyl fumarate Polpharma is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis.
Dimethyl fumarate Neuraxpharma is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis.
The physiological effects dimethyl fumarate has on the body is not well understood. It is known that dimethyl fumarate has anti-inflammatory and cytoprotective effects, which both are likely involved in its actions in multiple sclerosis patients.
Radiation-Sensitizing Agents
Drugs used to potentiate the effectiveness of radiation therapy in destroying unwanted cells. (See all compounds classified as Radiation-Sensitizing Agents.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
L04AX07
L04AX07
L04AX07
L04AX07
L04AX07
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AX - Other immunosuppressants
L04AX07 - Dimethyl fumarate
Absorption
Once ingested, dimethyl fumarate is rapidly hydroylyzed by esterases to MMF. Thus there is negligible amount of dimethyl fumarate in the body, and all pharmacokinetic information is quantified with MMF. In multiple sclerosis patients, the time to maximum concentration of MMF is 2 to 2.5 hours and the maximum concentration is 1.87 mg/L.
Route of Elimination
The main route of elimination is by CO2 exhalation that accounts for 60% of the dose. The other minor routes are through the kidney (16% metabolites and trace amounts of unchanged MMF) and the feces (1%).
Volume of Distribution
In healthy people, MMF has a variable volume of distribution of 53 to 73 litres.
Clearance
MMF clearance was not quantified.
After oral administration of Tecfidera, dimethyl fumarate undergoes rapid presystemic hydrolysis by esterases and is converted to its active metabolite, monomethyl fumarate (MMF). Dimethyl fumarate is not quantifiable in plasma following oral administration of Tecfidera. Therefore all pharmacokinetic analyses related to Tecfidera were performed with plasma MMF concentrations. ... The median Tmax of MMF is 2-2.5 hours. The peak plasma concentration (Cmax) and overall exposure (AUC) increased approximately dose proportionally in the dose range studied (120 mg to 360 mg). Following administration of Tecfidera 240 mg twice a day with food, the mean Cmax of MMF was 1.87 mg/L and AUC was 8.21 mg.hr/L in MS patients.
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
Exhalation of CO2 is the primary route of elimination, accounting for approximately 60% of the Tecfidera dose. Renal and fecal elimination are minor routes of elimination, accounting for 16% and 1% of the dose respectively. Trace amounts of unchanged monomethyl fumarate (MMF) were present in urine.
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
The apparent volume of distribution of monomethyl fumarate (MMF) varies between 53 and 73 L in healthy subjects. Human plasma protein binding of MMF is 27-45% and independent of concentration. /Monomethyl fumarate, active metabolite/
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
Dimethly fumarate is hydrozlied to its metabolite MMF in the GIT, tissues, and blood by esterases. MMF then undergoes subsequent metabolism in the tricarboxylic acid (TCA) cycle. Altogether the main metabolites formed are MMF, glucose, citric acid, and fumaric.
In humans, Tecfidera is extensively metabolized by esterases, which are ubiquitous in the gastrointestinal tract, blood and tissues, before it reaches the systemic circulation. Further metabolism occurs through the tricarboxylic acid (TCA) cycle, with no involvement of the cytochrome P450 (CYP) system. A single 240 mg (14)C-dimethyl fumarate dose study identified monomethyl fumarate, fumaric and citric acid, and glucose as the major metabolites in plasma. The downstream metabolism of fumaric and citric acid occurs through the TCA cycle, with exhalation of CO2 serving as a primary route of elimination. Less than 0.1% of the dose is excreted as unchanged dimethyl fumarate in urine.
Health Canada; Product Monograph for Tecfidera (Dimethyl Fumarate) Delayed-release Capsules, Drug Identification Number (DIN): 02404508 p.18 (Date of Revision: January 29, 2015). Available from, as of June 30, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
In humans, dimethyl fumarate is extensively metabolized by esterases, which are ubiquitous in the gastrointestinal tract, blood, and tissues, before it reaches the systemic circulation. Further metabolism of monomethyl fumarate (MMF) occurs through the tricarboxylic acid (TCA) cycle, with no involvement of the cytochrome P450 (CYP) system. MMF, fumaric and citric acid, and glucose are the major metabolites in plasma.
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
MMF has a short half life of about 1 hour, and MMF does not accumulate after repeated doses of dimethyl fumarate.
The terminal half-life of monomethyl fumarate (MMF) is approximately 1 hour and no circulating MMF is present at 24 hours in the majority of individuals. /Monomethyl fumarate, active metabolite/
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
The mechanism of action of dimethyl fumarate in multiple sclerosis is not well understood. It is thought to involve dimethyl fumarate degradation to its active metabolite monomethyl fumarate (MMF). MMF up-regulates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway that is activated in response to oxidative stress. As well MMF is an agonist at the nicotinic acid receptor, but the relevance of this is not known.
Dimethyl fumarate (DMF) is a fumaric acid ester that is used to treat psoriasis and multiple sclerosis. Recently, DMF was found to exhibit anti-tumor effects. However, the molecular mechanisms underlying these effects have not been elucidated. In this study, we investigated the mechanism of DMF-induced apoptosis in different human hematopoietic tumor cell lines. We found that DMF induced apoptosis in different human hematopoietic tumor cell lines but it did not affect the normal human B lymphocyte cell line RPMI 1788. We also observed a concurrent increase in caspase-3 activity and in the number of Annexin-V-positive cells. Furthermore, an examination of the survival signals, which are activated by apoptotic stimuli, revealed that DMF significantly inhibited nuclear factor-kB (NF-kB) p65 nuclear translocation. In addition, DMF suppressed B-cell lymphoma extra-large (Bcl-xL) and X-linked inhibitor of apoptosis (XIAP) expression whereas Bcl-2, survivin, Bcl-2-associated X protein (Bax), and Bim levels did not change. These results indicated that DMF induced apoptosis by suppressing NF-kB activation, and Bcl-xL and XIAP expression. These findings suggested that DMF might have potential as an anticancer agent that could be used in combination therapy with other anticancer drugs for the treatment of human hematopoietic tumors.
PMID:25443417 Tsubaki M et al; Biomed Pharmacother 68 (8): 999-1005 (2014)
Oxidative stress plays a crucial role in many neurodegenerative conditions such as Alzheimer's disease, amyotrophic lateral sclerosis and Parkinson's as well as Huntington's disease. Inflammation and oxidative stress are also thought to promote tissue damage in multiple sclerosis (MS). Recent data point at an important role of anti-oxidative pathways for tissue protection in chronic-progressive MS, particularly involving the transcription factor nuclear factor (erythroid-derived 2)-related factor 2 (Nrf2). ... In vitro, application of dimethylfumarate (DMF) leads to stabilization of Nrf2, activation of Nrf2-dependent transcriptional activity and abundant synthesis of detoxifying proteins. Furthermore, application of FAE involves direct modification of the inhibitor of Nrf2, Kelch-like ECH-associated protein 1. On cellular levels, the application of FAE enhances neuronal survival and protects astrocytes against oxidative stress. Increased levels of Nrf2 are detected in the central nervous system of DMF treated mice suffering from experimental autoimmune encephalomyelitis (EAE), an animal model of MS. In EAE, DMF ameliorates the disease course and improves preservation of myelin, axons and neurons. Finally, Nrf2 is also up-regulated in the spinal cord of autopsy specimens from untreated patients with MS, probably as part of a naturally occurring anti-oxidative response. In summary, oxidative stress and anti-oxidative pathways are important players in MS pathophysiology and constitute a promising target for future MS therapies like FAE.
PMID:23109883 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3472775 Lee DH et al; Int J Mol Sci 13 (9): 11783-803 (2012)
Multiple sclerosis (MS) is the most common multifocal inflammatory demyelinating disease of the central nervous system (CNS). Due to the progressive neurodegenerative nature of MS, developing treatments that exhibit direct neuroprotective effects are needed. Tecfidera (BG-12) is an oral formulation of the fumaric acid esters (FAE), containing the active metabolite dimethyl fumarate (DMF). Although BG-12 showed remarkable efficacy in lowering relapse rates in clinical trials, its mechanism of action in MS is not yet well understood. In this study, we reported the potential neuroprotective effects of dimethyl fumarate (DMF) on mouse and rat neural stem/progenitor cells (NPCs) and neurons. We found that DMF increased the frequency of the multipotent neurospheres and the survival of NPCs following oxidative stress with hydrogen peroxide (H2O2) treatment. In addition, utilizing the reactive oxygen species (ROS) assay, we showed that DMF reduced ROS production induced by H2O2. DMF also decreased oxidative stress-induced apoptosis. Using motor neuron survival assay, DMF significantly promoted survival of motor neurons under oxidative stress. We further analyzed the expression of oxidative stress-induced genes in the NPC cultures and showed that DMF increased the expression of transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) at both levels of RNA and protein. Furthermore, we demonstrated the involvement of Nrf2-ERK1/2 MAPK pathway in DMF-mediated neuroprotection. Finally, we utilized SuperArray gene screen technology to identify additional anti-oxidative stress genes (Gstp1, Sod2, Nqo1, Srxn1, Fth1). Our data suggests that analysis of anti-oxidative stress mechanisms may yield further insights into new targets for treatment of multiple sclerosis (MS).
PMID:26090715 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4490529 Wang Q et al; Int J Mol Sci 16 (6): 13885-13907 (2015)
The mechanism by which dimethyl fumarate (DMF) exerts its therapeutic effect in multiple sclerosis is unknown. DMF and the metabolite, monomethyl fumarate (MMF), have been shown to activate the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in vitro and in vivo in animals and humans. The Nrf2 pathway is involved in the cellular response to oxidative stress. MMF has been identified as a nicotinic acid receptor agonist in vitro.
NIH; DailyMed. Current Medication Information for Tecfidera (Dimethyl Fumarate) Capsule (Updated: April 2015). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=665d7e74-036c-5f68-5b67-ab84b9b49151
For more Mechanism of Action (Complete) data for DIMETHYL FUMARATE (14 total), please visit the HSDB record page.