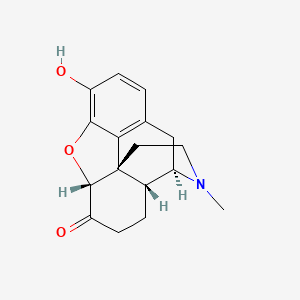

1. Dihydromorphinone
2. Dilaudid
3. Hydromorphon
4. Hydromorphone Hydrochloride
5. Laudacon
6. Palladone
1. Dihydromorphinone
2. Dimorphone
3. Hydromorphon
4. Idromorfone
5. Novolaudon
6. 466-99-9
7. Dimo
8. 7,8-dihydromorphinone
9. Dihydromorfinon
10. Hydromorfona
11. Dilaudid
12. Hydromorfona [spanish]
13. Dihydromorfinon [czech]
14. Hidromorfona
15. Hydromorphonum
16. Hidromorfona [inn-spanish]
17. Hydromorphonum [inn-latin]
18. Palladone
19. 6-deoxy-7,8-dihydro-6-oxomorphine
20. 4,5-epoxy-3-hydroxy-17-methylmorphinan-6-one
21. Dilaudid-hp
22. 4,5alpha-epoxy-3-hydroxy-17-methyl-6-morphinanone
23. Jurnista
24. (-)-(5r)-4,5-epoxy-3-hydroxy-9alpha-methylmorphinan-6-one
25. Morphinone, Dihydro-
26. Laudacon
27. Hydromorphone (inn)
28. N02aa03
29. Nsc-19046
30. Dilaudid Oros
31. Chebi:5790
32. Chembl398707
33. Ids-nh-004
34. (-)-hydromorphone
35. 3-hydroxy-17-methyl-4,5alpha-epoxymorphinan-6-one
36. Dea No. 9150
37. Idromorfone [dcit]
38. Laudicon
39. Nsc 19046
40. Q812464r06
41. Hydromorphone [inn]
42. (4r,4ar,7ar,12bs)-9-hydroxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one
43. Hydromorphone [inn:ban]
44. (4r,4ar,7ar,12bs)-9-hydroxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
45. Hsdb 3341
46. Einecs 207-383-5
47. Morphinan-6-one, 4,5alpha-epoxy-3-hydroxy-17-methyl-
48. Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, (5.alpha.)-
49. Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, (5alpha)-
50. Unii-q812464r06
51. Hydromorphone Form I
52. (5alpha)-3-hydroxy-17-methyl-4,5-epoxymorphinan-6-one
53. Hydromorphone Form Ii
54. Morphinan-6-one, 4,5-alpha-epoxy-3-hydroxy-17-methyl-
55. 4,5.alpha.-epoxy-3-hydroxy-17-methylmorphinan-6-one
56. Hydromorphone [mi]
57. Schembl2255
58. Hydromorphone [hsdb]
59. Hydromorphone [vandf]
60. Hydromorphone [who-dd]
61. Gtpl7082
62. Dtxsid8023133
63. Zinc402954
64. Nsc19046
65. Bdbm50241341
66. Hydromorphone 0.1 Mg/ml In Methanol
67. Hydromorphone 1.0 Mg/ml In Methanol
68. Db00327
69. C07042
70. D08047
71. Q303646
72. Morphinan-6-one,5.alpha.-epoxy-3-hydroxy-17-methyl-
73. Morphinan-6-one,5-epoxy-3-hydroxy-17-methyl-, (5.alpha.)-
74. Wln: T B6566 B6/co 4abbc R Bx Fv Ho Pn Ght&&ttj Jq P1
75. Hydrocodone Hydrogen Tartrate 2.5-hydrate Impurity K [ep Impurity]
76. Hydromorphone Solution, 1 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
77. (1s,5r,13r,17r)-10-hydroxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0?,??.0?,??.0?,??]octadeca-7,9,11(18)-trien-14-one
78. (1s,5r,13r,17r)-10-hydroxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10-trien-14-one
79. (4r,4ar,7ar,12bs)-9-hydroxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one]
| Molecular Weight | 285.34 g/mol |
|---|---|
| Molecular Formula | C17H19NO3 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 285.13649347 g/mol |
| Monoisotopic Mass | 285.13649347 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 494 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | DILAUDID-HP |
| Active Ingredient | HYDROMORPHONE HYDROCHLORIDE |
| Company | FRESENIUS KABI USA (Application Number: N019034. Patents: 6589960, 9248229, 9731082) |
| 2 of 4 | |
|---|---|
| Drug Name | EXALGO |
| Active Ingredient | HYDROMORPHONE HYDROCHLORIDE |
| Company | SPECGX LLC (Application Number: N021217) |
| 3 of 4 | |
|---|---|
| Drug Name | HYDROMORPHONE HYDROCHLORIDE |
| Active Ingredient | HYDROMORPHONE HYDROCHLORIDE |
| Company | ACTAVIS LABS FL INC (Application Number: A202144); AKORN (Application Number: A078228); AKORN (Application Number: A078261); ASCENT PHARMS INC (Application Number: A210176); ASCENT PHARMS INC (Application Number: A210506); AUROLIFE PHARMA LLC (Application Number: A205814); BARR (Application Number: A076444); ELITE LABS (Application Number: A076723); EUROHLTH INTL SARL (Application Number: A202159); HOSPIRA INC (Application Number: A078591); HOSPIRA INC (Application Number: N200403); LANNETT CO INC (Application Number: A077471); OSMOTICA (Application Number: A205629); PADDOCK LLC (Application Number: A204278); SPECGX LLC (Application Number: A076855); WEST-WARD PHARMS INT (Application Number: A074597); WEST-WARD PHARMS INT (Application Number: A074653) |
| 4 of 4 | |
|---|---|
| Drug Name | DILAUDID |
| Active Ingredient | HYDROMORPHONE HYDROCHLORIDE |
| Company | FRESENIUS KABI USA (Application Number: N019034. Patents: 6589960, 9248229, 9731082); RHODES PHARMS (Application Number: N019891. Patent: 6589960); RHODES PHARMS (Application Number: N019892. Patent: 6589960) |
Analgesics, Opioid; Narcotics
National Library of Medicine's Medical Subject Headings. Hydromorphone. Online file (MeSH, 2016). Available from, as of August 12, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Hydromorphone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=hydromorphone&Search=Search
Hydromorphone hydrochloride may be administered by subcutaneous, IM, or slow IV injection; the drug also may be administered orally as conventional (immediate-release) or extended-release tablets or as an oral solution. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2218
Hydromorphone hydrochloride is used for the relief of moderate to severe pain and for the relief of non-productive cough. /Hydromorphone hydrochloride/
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 64
For more Therapeutic Uses (Complete) data for HYDROMORPHONE (12 total), please visit the HSDB record page.
The U.S. Food and Drug Administration (FDA) is warning about several safety issues with the entire class of opioid pain medicines. These safety risks are potentially harmful interactions with numerous other medications, problems with the adrenal glands, and decreased sex hormone levels. We are requiring changes to the labels of all opioid drugs to warn about these risks. Opioids can interact with antidepressants and migraine medicines to cause a serious central nervous system reaction called serotonin syndrome, in which high levels of the chemical serotonin build up in the brain and cause toxicity. Taking opioids may lead to a rare, but serious condition in which the adrenal glands do not produce adequate amounts of the hormone cortisol. Cortisol helps the body respond to stress. Long-term use of opioids may be associated with decreased sex hormone levels and symptoms such as reduced interest in sex, impotence, or infertility.
FDA; FDA Drug Safety Communication: FDA Warns About Several Safety Issues with Opioid Pain Medicines; Requires Label Changes (March 22, 2016). Available from, as of March 22, 2016: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery
In a continuing effort to educate prescribers and patients about the potential risks related to opioid use, the U.S. Food and Drug Administration today announced required class-wide safety labeling changes for immediate-release (IR) opioid pain medications. Among the changes, the FDA is requiring a new boxed warning about the serious risks of misuse, abuse, addiction, overdose and death. Today's actions are among a number of steps the agency recently outlined in a plan to reassess its approach to opioid medications. The plan is focused on policies aimed at reversing the epidemic, while still providing patients in pain access to effective relief.
FDA; FDA News Release: FDA Announces Enhanced Warnings for Immediate-Release Opioid Pain Medications Related to Risks of Misuse, Abuse, Addiction, Overdose and Death (March 22, 2016). Available from, as of March 22, 2016 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491739.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery
CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016; This guideline provides recommendations for primary care clinicians who are prescribing opioids for chronic pain outside of active cancer treatment, palliative care, and end-of-life care. The guideline addresses 1) when to initiate or continue opioids for chronic pain; 2) opioid selection, dosage, duration, follow-up, and discontinuation; and 3) assessing risk and addressing harms of opioid use. CDC developed the guideline using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, and recommendations are made on the basis of a systematic review of the scientific evidence while considering benefits and harms, values and preferences, and resource allocation. CDC obtained input from experts, stakeholders, the public, peer reviewers, and a federally chartered advisory committee. It is important that patients receive appropriate pain treatment with careful consideration of the benefits and risks of treatment options. This guideline is intended to improve communication between clinicians and patients about the risks and benefits of opioid therapy for chronic pain, improve the safety and effectiveness of pain treatment, and reduce the risks associated with long-term opioid therapy, including opioid use disorder, overdose, and death.
Dowell D et al; Morbidity and Mortality Weekly Report (MMWR) 65 (1):1-49 (2016); Available from, as of March 22, 2016: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm
The incidence of all adverse effects was 18% with hydromorphone compared with 6% of morphine, 4% of methadone, and 4% of codeine recipients. Seizures and myoclonus have occurred with high parenteral doses. Nausea and vomiting are common with oral dosing, and constipation occurred in 40% of patients receiving it parenterally. Ureteral spasm is reported. When given by continuous SQ infusion, local edema, inflammation, and infection may occur. ... Withdrawal symptoms may occur in dependent individuals if used with opioid agonists or agonist/antagonist agents.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 764
For more Drug Warnings (Complete) data for HYDROMORPHONE (12 total), please visit the HSDB record page.
Two fatalities exhibited postmortem blood hydromorphone concentrations of 0.5 and 1.2 mg/L, respectively. Lethal blood concentrations of hydromorphone (more than 0.01 mg/dL) were present in 12 individuals who died from the IV use of Dilaudid.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 764
Toxic hydromorphone blood concentration: 10-200 ug/dL; Lethal hydromorphone blood concentration: >300 ug/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 298
Hydromorphone is indicated for the management of moderate to severe acute pain and severe chronic pain. Due to its addictive potential and overdose risk, hydromorphone is only prescribed when other first-line treatments have failed. The WHO has proposed a three-step ladder for the management of pain in which it is suggested to start with a non-opioid medication followed by addition of weak opioids to the non-opioid treatment for moderate pain and finishing in the use of strong opioids such as hydromorphone along with the existing regimen for cases of severe pain. Off-label, hydromorphone can be administered for the suppression of refractory cough.
FDA Label
In clinical trials, hydromorphone has been shown to be suitable for pain relief in patients that do not tolerate the side effects of [morphine] or that suffer from renal failure or asthma. It has been shown to be 5-7 times more potent than morphine with a shorter duration of analgesia. Some of the observed effects of the consumption of hydromorphone for acute pain are complete and longlasting pain relief when compared to other pain relief agents such as [meperidine], [morphine], [diamorphine], [bupivacaine], [indomethacin], and [fentanyl]. On the same trials, hydromorphone was shown to produce respiratory depression, lower cognitive function, miosis, mydriasis, constipation, hypotension, and vertigo but to present a reduced incidence of pruritus (which indicates a lower release of histamine) and nausea. The respiratory depression is known to be caused by the effect on the brain stem respiratory centers as well as to a reduction in the responsiveness of this brain stems to increase carbon dioxide tension.
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
N02AA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N02 - Analgesics
N02A - Opioids
N02AA - Natural opium alkaloids
N02AA03 - Hydromorphone
Absorption
The immediate release version of hydromorphone reaches its peak concentration after 30-60 minutes while the extended-release version reaches the peak concentration after 9 hours. When administered orally, hydromorphone is absorbed mainly in the upper small intestine with a bioavailability of 60% due to intensive first-pass metabolism. In the controlled-release version of hydromorphone, the absorption follows a biphasic pharmacokinetic profile. However, even though there are clear distinctions in the absorption pathway of hydromorphone, the AUC of both versions is reported to be of 34 ng.h/ml which indicates an equivalence. The parenteral administration of hydromorphone, which is the most common pathway, presents an almost immediate absorption as observed by the presence of peak plasma concentration almost immediately. This peak plasma concentration declines rapidly due to fast redistribution into liver, spleen, kidney and skeletal muscle. In the parenteral route, the pharmacokinetic profile is log-linear and dose-dependent and to present a higher bioavailability of 78%. Other administration routes such as rectal, nasal, intraspinal and transdermal present lower bioavailability and changes in their pharmacokinetic profile.
Route of Elimination
The main elimination route of hydromorphone is through the urine in the form of the main metabolite hydromorphone-3-glucuronide. The elimination of the parent compound represents 7% of the urine elimination and 1% of the fecal elimination.
Volume of Distribution
The volume of distribution of hydromorphone is reported to be of 4 L/kg.
Clearance
The mean plasma clearance of hydromorphone is reported to be of 105.7 ml/min. The systemic clearance is reported to be of 1.96 L/min.
Hydromorphone hydrochloride is rapidly but incompletely absorbed from the gastrointestinal tract after oral doses; peak plasma concentrations occur within 0.5 to 1 hour. Oral bioavailability is about 50% as it undergoes extensive first-pass metabolism. Hydromorphone is about 8 to 19% bound to plasma proteins. A plasma elimination half-life of about 2.5 hours has been reported after oral or intravenous doses. Hydromorphone appears to be widely distributed in the tissues; it crosses the placenta and is distributed into breast milk. It is extensively metabolized by glucuronidation in the liver and excreted in the urine mainly as conjugated hydromorphone, dihydroisomorphine, and dihydromorphine. /Hydromorphone hydrochloride/
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 64
Hydromorphone hydrochloride is well absorbed following oral, rectal, or parenteral administration. Hydromorphone has a more rapid onset and may have a shorter duration of action than does morphine. The onset of action of hydromorphone with conventional (immediate-release) preparations is usually 15-30 minutes and analgesia is maintained for 4-5 hours, depending on the route of administration. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2220
/MILK/ Because some opiate agonists have been detected in milk, the manufacturers state that women should not breast-feed while receiving hydromorphone hydrochloride. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2220
Hydromorphone is ... excreted principally in the urine as the glucuronide conjugate. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2220
For more Absorption, Distribution and Excretion (Complete) data for HYDROMORPHONE (19 total), please visit the HSDB record page.
The metabolism of hydromorphone is mainly hepatic and it is represented by the generation of hydromorphone-3-glucuronide through glucuronidation reactions. This primary metabolic pathway is done by the activity of the UDP-glucuronosyltransferase-2B7. The first-pass hepatic metabolism is so large that it represents 62% of the initial administered dose. On the other hand, hydromorphone is also characterized by the presence of minor metabolic pathways such as the CYP3A4- and CYP2C9-driven generation of norhydromorphone.
Metabolism is primarily via the liver, with metabolites and unchanged drug excreted in the urine.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 764
Hydromorphone is metabolized primarily in the liver where it undergoes conjugation with glucuronic acid and is excreted principally in the urine as the glucuronide conjugate. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2220
Pain is one the most common symptoms experienced by palliative care patients. The treatment of pain involves the use of strong opioids such as hydromorphone, morphine, methadone, fentanyl, oxycodone, oxymorphone, or levorphanol for moderate to severe pain. Hydromorphone is metabolized by the liver to hydromorphone-3-glucuronide (H3G), a compound that can potentially cause neuroexcitatory phenomena with accumulation. Pharmacokinetic studies have shown that H3G levels in patients with renal insufficiency are 4 times as high as those with normal renal function; however, reports have been conflicting as to whether or not it is safe to use hydromorphone in renal insufficiency.
PMID:21823925 Paramanandam G et al; J Palliat Med 14 (9): 1029-33 (2011)
Morphine is one of several opioids used to treat chronic pain. Because of its high abuse potential, urine drug tests can confirm "consistency with prescribed medications." Hydromorphone is a recently described minor metabolite of morphine, but few data exist on the characteristics of this metabolic pathway or the relationship of morphine and hydromorphone between and within subjects. Part I of this retrospective study shows that formation of hydromorphone from morphine is concentration-dependent and possibly saturated at high concentrations of morphine. In addition, the percentage of ultra-rapid metabolizers and poor metabolizers can be determined using the lower asymptote of a sigmoidal mathematical fit and are estimated to be 0.63 and 4.0%, respectively. Expected limits of morphine and hydromorphone (as a result of morphine metabolism) concentrations in the urine were established. Part II of this study used the metabolic ratio (hydromorphone-morphine) to determine the inter-patient and intra-patient variability in morphine metabolism to hydromorphone. Metabolic ratio values varied over a large range; 25-fold and 7-fold, respectively. The expected limits established in this study can assist in assessing the cause for possible variances in metabolism, such as drug interactions. The wide variability between and within subjects may explain unpredictable, adverse effects.
PMID:22511699 Hughes MM et al; J Anal Toxicol 36 (4): 250-6 (2012)
For more Metabolism/Metabolites (Complete) data for HYDROMORPHONE (8 total), please visit the HSDB record page.
Hydromorphone has known human metabolites that include Hydromorphone 3-beta-O-glucuronide, Hydromorphone 3-sulfate, and Norhydromorphone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of hydromorphone immediate-release is of 2-3 hour while the extended release can range from 8-15 hours.
The parent drug has a half-life of elimination of 2.5 hours.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 764
The terminal elimination half-life of hydromorphone after IV administration is about 2.3 hours. /Hydromorphone hydrochloride/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2220
/In beagle dogs/, the serum half-life after SC hydromorphone at 0.1 mg kg(-1) and 0.5 mg kg(-1) was 0.66 hours and 1.11 hours, respectively. Hydromorphone has a short half-life, suggesting that frequent dosing intervals are needed. Based on pharmacokinetic parameters calculated in this study, 0.1 mg kg(-1) IV or SC q 2 hours or a constant rate infusion of hydromorphone at 0.03 mg kg(-1) hour(-1) are suggested for future studies to assess the analgesic effect of hydromorphone.
PMID:18282253 Kukanich B et al; Vet Anaesth Analg (Epub ahead of print) (2008)
The purpose of this study was to determine the pharmacokinetics of hydrocodone and its active metabolite hydromorphone in six healthy Greyhound dogs. Hydrocodone bitartrate was administered at a targeted dose of 0.5 mg/kg PO. ... The mean hydromorphone CMAX was 5.2 ng/mL at 1.37 hr with a terminal half-life of 3.07 hr. ...
PMID:23098635 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4219551 KuKanich B, Spade J; Vet J 196 (2): 266-8 (2013)
For more Biological Half-Life (Complete) data for HYDROMORPHONE (6 total), please visit the HSDB record page.
Hydromorphone is an opioid agonist that can bind to different types of opioid receptors. Its analgesic effect is suggested to be related to the effect on the mu-opioid receptors. It has been reported to also have a minor affinity for the delta and kappa receptor. On the other hand, it is known to act at the level of the medulla which allows it to depress the respiratory drive and suppress cough. The onset of action of the immediate release form of hydromorphone is achieved in 15-20 minutes and having a lasting effect for 3-4 hours while the extended-release form onset of action is of 6 hours lasting for about 13 hours.
/Accumulation of the active morphine-3-glucuronide (M3G) and hydromorphone-3-glucuronide (H3G) metabolites is one proposed mechanism for the development of neuroexcitatory effects including allodynia and opioid-induced hyperalgesia (OIH).
PMID:22925158 Juba KM et al; J Palliat Med 16 (7): 809-12 (2013)