
1. Alpha, Pge2
2. Alpha, Prostaglandin E2
3. E2 Alpha, Prostaglandin
4. E2, Prostaglandin
5. E2alpha, Prostaglandin
6. Gel, Prepidil
7. Pge2
8. Pge2 Alpha
9. Pge2alpha
10. Prepidil Gel
11. Prostaglandin E2
12. Prostaglandin E2 Alpha
13. Prostaglandin E2alpha
14. Prostenon
1. Prostaglandin E2
2. Pge2
3. 363-24-6
4. Prostin E2
5. Prepidil
6. Minprostin E2
7. Cervidil
8. Propess
9. Minprositin E2
10. Dinoproston
11. Prostin
12. (15s)-prostaglandin E2
13. Prostarmon E
14. Dinoprostonum
15. L-prostaglandin E2
16. Dinoprostona
17. Glandin
18. Enzaprost E
19. L-pge2
20. (5z,11alpha,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
21. U-12062
22. (e,z)-(1r,2r,3r)-7-(3-hydroxy-2-((3s)-(3-hydroxy-1-octenyl))-5-oxocyclopentyl)-5-heptenoic Acid
23. (-)-prostaglandin E2
24. Prostaglandin E
25. Nsc 165560
26. [3h]pge2
27. U 12062
28. [3h]prostaglandin E2
29. (z)-7-((1r,2r,3r)-3-hydroxy-2-((s,e)-3-hydroxyoct-1-en-1-yl)-5-oxocyclopentyl)hept-5-enoic Acid
30. (z)-7-[(1r,2r,3r)-3-hydroxy-2-[(e,3s)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]hept-5-enoic Acid
31. Chembl548
32. Nsc 196514
33. Nsc-165560
34. Nsc-196514
35. K7q1jqr04m
36. 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic Acid
37. L-7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic Acid
38. (5z,13e)-(15s)-11alpha,15-dihydroxy-9-oxoprost-13-enoate
39. Chebi:15551
40. (5z,13e)-(15s)-11alpha,15-dihydroxy-9-oxoprosta-5,13-dienoate
41. U-12,062
42. 5-heptenoic Acid, 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-
43. 5-heptenoic Acid, 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-, L-
44. Pge2alpha
45. Prosta-5,13-dien-1-oic Acid, 11,15-dihydroxy-9-oxo-, (5z,11alpha,13e,15s)-
46. Prostaglandin E(2)
47. (z)-7-[(1r,2r,3r)-3-hydroxy-2-[(e,3s)-3-hydroxyoct-1-enyl]-5-oxo-cyclopentyl]hept-5-enoic Acid
48. Ncgc00092361-05
49. Prostaglandin E2alpha
50. Prostaglandin E2.alpha.
51. (5z,13e,15s)-11alpha,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
52. Dsstox_cid_2947
53. Dsstox_rid_76801
54. Dsstox_gsid_22947
55. Dinoprostonum [inn-latin]
56. 9-oxo-11r,15s-dihydroxy-5z,13e-prostadienoic Acid
57. Dinoprostona [inn-spanish]
58. Cerviprime
59. Prostenone
60. 7-[3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl]-5-heptenoic Acid
61. Prostarmon E2
62. Prosta-5,13-dien-1-oic Acid, 11,15-dihydroxy-9-oxo-, (5z,11.alpha.,13e,15s)-
63. Cervidil (tn)
64. Prepidil (tn)
65. Cas-363-24-6
66. Dinoprostone Prostaglandin E2
67. U,062
68. Prostin E2 (tn)
69. Pge2-[3,3,4,4-d4]
70. Sr-05000001459
71. Einecs 206-656-6
72. Unii-k7q1jqr04m
73. Dinoprostone (jan/usp/inn)
74. (5z,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
75. Bml1-f07
76. Prosta-5, (5z,11.alpha.,13e,15s)-11,15-dihydroxy-9-oxo-
77. Prosta-5, 11,15-dihydroxy-9-oxo-, (5z,11.alpha.,13e,15s)-
78. Prosta-5, 11,5-dihydroxy-9-oxo-, (5z,11.alpha.,13e,15s)-
79. [3h]dinoprostone
80. 5-heptenoic Acid, 7-[3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl]-
81. Prosta-glandin E2
82. Ncgc00092361-06
83. (e,2r,3r)-7-[3-hydroxy-2-[(3s)-(3-hydroxy-1-octenyl)]-5-oxocyclopentyl]-5-heptenoic Acid
84. P2e
85. Prestwick_793
86. (5z,11-alpha,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
87. Mfcd00077861
88. [3h]prostin E2
89. Bms-279654 & Pge2
90. Dinoprostone Beta-cyclodextrin Clathrate
91. Dinoprostone [usan:usp:inn:ban:jan]
92. (5z,11alpha,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic
93. Dinoprostone [inn]
94. Dinoprostone [jan]
95. Dinoprostone [usan]
96. (+/-)-prostaglandin E2
97. Prostaglandin E2 (pge2)
98. Dinoprostone [vandf]
99. Schembl25533
100. Bspbio_001490
101. Dinoprostone [mart.]
102. Dinoprostone [usp-rs]
103. Dinoprostone [who-dd]
104. Prostaglandin E2 [mi]
105. Gtpl1883
106. Gtpl1916
107. Dtxsid4022947
108. [3h]-pge2
109. Bdbm35847
110. Dtxsid00859353
111. Dinoprostone [orange Book]
112. Hms1361k12
113. Hms1791k12
114. Hms1989k12
115. Hms2089d17
116. Hms3268j12
117. Hms3402k12
118. Hms3413c20
119. Hms3648f07
120. Hms3677c20
121. Dinoprostone [ep Monograph]
122. Dinoprostone [usp Impurity]
123. (z)-7-((1r,2r,3r)-3-hydroxy-2-((s,e)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)hept-5-enoic Acid
124. Amy30102
125. Ex-a1773
126. Zinc3830713
127. Dinoprostone [usp Monograph]
128. Tox21_111196
129. Lmfa03010003
130. Nsc165560
131. Nsc196514
132. S3003
133. (5z,11.alpha.,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
134. Akos015920228
135. Tox21_111196_1
136. Ac-6098
137. Ccg-208092
138. Ccg-208256
139. Cs-6932
140. Db00917
141. Idi1_033960
142. Prosta-5,13-dien-1-oic Acid, (5z,11-alpha,13e,15s)-11,15-dihydroxy-9-oxo-
143. Smp2_000056
144. Ncgc00092361-01
145. Ncgc00092361-02
146. Ncgc00092361-03
147. Ncgc00092361-04
148. As-71847
149. Dinoprostone 100 Microg/ml In Acetonitrile
150. Hy-101952
151. Alprostadil Impurity G [ep Impurity]
152. B7005
153. P1884
154. C00584
155. D00079
156. Prostaglandin E2, >=93% (hplc), Synthetic
157. 363p246
158. A823207
159. Q416554
160. Sr-01000946417
161. J-502620
162. Sr-01000946417-1
163. Sr-05000001459-1
164. Sr-05000001459-3
165. Sr-05000001459-4
166. Brd-k26521938-001-04-9
167. 05d31bd5-818b-4a92-8cfc-bec19926a5b3
168. 2-amino-1-benzo[1,3]dioxol-5-yl-ethanonehydrochloride
169. Dinoprostone, European Pharmacopoeia (ep) Reference Standard
170. (5z,13e)-(15s)-11alpha,15-dihydroxy-9-oxoprost-13-enoic Acid
171. (5z,13e,15s)-11-alpha,15-dihydroxy-9-oxoprost-5,13-dienoate
172. Dinoprostone, United States Pharmacopeia (usp) Reference Standard
173. (5z,11?,13e,15s)-11,15-dihydroxy-9-oxo-prosta-5,13-dien-1oic Acid
174. (5z,11i+/-,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dienoic Acid
175. (5z,13e)-(15s)-11alpha,15-dihydroxy-9-oxoprosta-5,13-dienoic Acid
176. (5z,13e,15s)-11-alpha,15-dihydroxy-9-oxoprost-5,13-dienoic Acid
177. 5z,11alpha,13e,15s)-11,15-dihydroxy-9-oxo-rosta-5,13-dien-1-oic Acid
178. Prostaglandin E2, Synthetic, Powder, Bioreagent, Suitable For Cell Culture
179. (5z,11alpha,12alpha,13e,15s)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic Acid
180. (5z,11alpha,13e,15s)-11,15-dihydroxy-9-o Xo-prosta-5,13-dien-1oic Acid
181. Prostaglandin E2, Gamma-irradiated, Powder, Bioxtra, Suitable For Cell Culture
182. (5z)-7-[(1r,2r,3r)-3-hydroxy-2-[(1e,3s)-3-hydroxyoct-1-en-1-yl]-5-oxocyclopentyl]hept-5-enoic Acid
183. 22230-04-2
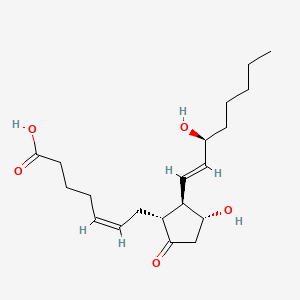
| Molecular Weight | 352.5 g/mol |
|---|---|
| Molecular Formula | C20H32O5 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 12 |
| Exact Mass | 352.22497412 g/mol |
| Monoisotopic Mass | 352.22497412 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 469 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Cervidil |
| Drug Label | Dinoprostone vaginal insert is a thin, flat, polymeric slab which is rectangular in shape with rounded corners contained within the pouch of an off-white knitted polyester retrieval system. Each slab is buff colored, semitransparent and contains 10 m... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Insert, extended release |
| Route | Vaginal |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Ferring Controlled |
| 2 of 6 | |
|---|---|
| Drug Name | Prepidil |
| PubMed Health | Dinoprostone (Vaginal) |
| Drug Classes | Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | PREPIDIL Gel contains dinoprostone as the naturally occurring form of prostaglandin E2 (PGE2) and is designated chemically as (5Z, 11a, 13E, 15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid. The molecular formula is C20H32O5 and the molecular w... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Gel |
| Route | Endocervical |
| Strength | 0.5mg/3gm |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 3 of 6 | |
|---|---|
| Drug Name | Prostin e2 |
| PubMed Health | Dinoprostone (Vaginal) |
| Drug Classes | Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | PROSTIN E2 Vaginal Suppository, an oxytocic, contains dinoprostone as the naturally occurring prostaglandin E2 (PGE2).Its chemical name is (5Z,11,13E,15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid and the structural formula is represented b... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Suppository |
| Route | Vaginal |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 4 of 6 | |
|---|---|
| Drug Name | Cervidil |
| Drug Label | Dinoprostone vaginal insert is a thin, flat, polymeric slab which is rectangular in shape with rounded corners contained within the pouch of an off-white knitted polyester retrieval system. Each slab is buff colored, semitransparent and contains 10 m... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Insert, extended release |
| Route | Vaginal |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Ferring Controlled |
| 5 of 6 | |
|---|---|
| Drug Name | Prepidil |
| PubMed Health | Dinoprostone (Vaginal) |
| Drug Classes | Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | PREPIDIL Gel contains dinoprostone as the naturally occurring form of prostaglandin E2 (PGE2) and is designated chemically as (5Z, 11a, 13E, 15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid. The molecular formula is C20H32O5 and the molecular w... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Gel |
| Route | Endocervical |
| Strength | 0.5mg/3gm |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 6 of 6 | |
|---|---|
| Drug Name | Prostin e2 |
| PubMed Health | Dinoprostone (Vaginal) |
| Drug Classes | Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | PROSTIN E2 Vaginal Suppository, an oxytocic, contains dinoprostone as the naturally occurring prostaglandin E2 (PGE2).Its chemical name is (5Z,11,13E,15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid and the structural formula is represented b... |
| Active Ingredient | Dinoprostone |
| Dosage Form | Suppository |
| Route | Vaginal |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
For the termination of pregnancy during the second trimester (from the 12th through the 20th gestational week as calculated from the first day of the last normal menstrual period), as well as for evacuation of the uterine contents in the management of missed abortion or intrauterine fetal death up to 28 weeks of gestational age as calculated from the first day of the last normal menstrual period. Also used in the management of nonmetastatic gestational trophoblastic disease (benign hydatidiform mole). Other indications include improving the cervical inducibility (cervical "ripening") in pregnant women at or near term with a medical or obstetrical need for labor induction, and the management of postpartum hemorrhage.
Dinoprostone is equivalent to prostaglandin E2 (PGE2). It stimulates labor and delivery by stimulating the uterine, and thus terminates pregnancy. Dinoprostone is also capable of stimulating the smooth muscle of the gastrointestinal tract of man. This activity may be responsible for the vomiting and/or diarrhea that is not uncommon when dinoprostone is used to terminate pregnancy.
Oxytocics
Drugs that stimulate contraction of the myometrium. They are used to induce LABOR, OBSTETRIC at term, to prevent or control postpartum or postabortion hemorrhage, and to assess fetal status in high risk pregnancies. They may also be used alone or with other drugs to induce abortions (ABORTIFACIENTS). Oxytocics used clinically include the neurohypophyseal hormone OXYTOCIN and certain prostaglandins and ergot alkaloids. (From AMA Drug Evaluations, 1994, p1157) (See all compounds classified as Oxytocics.)
G02AD02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02A - Uterotonics
G02AD - Prostaglandins
G02AD02 - Dinoprostone
Absorption
Absorbed at a rate of 0.3 mg per hour over 12 hours while the vaginal system is in place.
Route of Elimination
The major route of elimination of the products of PGE2 metabolism is the kidneys.
Rapid metabolism of dinoprostone occurs primarily in the local tissues; any systemic absorption of the medication is cleared mainly in the maternal lungs and, secondarily, at sites such as the liver and kidneys.
Less than 5 minutes.
Dinoprostone administered intravaginally stimulates the myometrium of the gravid uterus to contract in a manner that is similar to the contractions seen in the term uterus during labor, resulting in the evacuation of the products of conception from the uterus. It is believed that dinoprostone exerts its uterine effects via direct myometrial stimulation, but the exact mechanism of action is unkown. Other suggested mechanisms include the regulation of cellular membrane calcium transport and of intracellular concentrations of cyclic 3',5'-adenosine monophosphate. Dinoprostone also appears to produce local cervical effects including softening, effacement, and dilation. The exact mechanism of action for this effect is also unknown, but it has been suggested that this effect may be associated with collagen degradation caused by secretion of the enzyme collagenase as a partial response to locally administered dinoprostone.