


1. (+)-dipivefrin
2. (-)-dipivefrin
3. (r)-dipivefrine
4. (rs)-4-(1-hydroxy-2-(methylamino)ethyl)-o-phenylene Dipivalate Hydrochloride
5. (s)-dipivefrin
6. (s)-dipivefrine
7. Adrenaline Dipivalate
8. Akpro
9. Apo-dipivefrin
10. D Epifrin
11. Diopine
12. Dipivaloylepinephrine
13. Dipivalyl Epinephrine
14. Dipivefrin Acetate, (+-)-isomer
15. Dipivefrin Citrate (1:1), (+-)-isomer
16. Dipivefrin Hcl
17. Dipivefrin Hydrochloride
18. Dipivefrin Hydrochloride, (+-)-isomer
19. Dipivefrin Hydrochloride, (r)-
20. Dipivefrin Hydrochloride, (s)-
21. Dipivefrin Monophosphate, (+-)-isomer
22. Dipivefrin Monosulfate, (+-)-isomer
23. Dipivefrin Nitrate, (+-)-isomer
24. Dipivefrin Perchlorate
25. Dipivefrin Propanoate, (+-)-isomer
26. Dipivefrin Tartrate (1:1), (+-)-(r-(r*,r*))-isomer
27. Dipivefrin Tartrate (1:1), (r)-(r-(r*,r*))-isomer
28. Dipivefrin, (r)-isomer
29. Dipivefrine
30. Dipivefrine Hydrochloride
31. Dipivefrine Hydrochloride, (r)-
32. Dipivefrine Hydrochloride, (s)-
33. Dipivefrine, (r)-
34. Dipoquin
35. Glaucothil
36. Glaudrops
37. Pms-dipivefrin
38. Propanoic Acid, 2,2-dimethyl-, 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Ester, (+-)-
39. Propanoic Acid, 2,2-dimethyl-, 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Ester, (r)-
40. Propanoic Acid, 2,2-dimethyl-, 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Ester, (s)-
41. Propine
42. Ratio-dipivefrin
1. Dipivefrine
2. 52365-63-6
3. Dipivefrinum
4. Dipivalyl Epinephrine
5. Dipivefrin [usan]
6. Dipivefrina
7. Dipivefrine (inn)
8. Dipivefrine [inn]
9. Dipivefrin (usan)
10. 8q1pvl543g
11. Chebi:4646
12. 1-(3',4'-dipivaloyloxyphenyl)-2-methylamino-1-ethanol
13. 4-[1-hydroxy-2-(methylamino)ethyl]-o-phenylene Divavalate
14. Dipivefrinum [inn-latin]
15. Dipivefrina [inn-spanish]
16. 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Bis(2,2-dimethylpropanoate)
17. K 30081
18. Unii-8q1pvl543g
19. 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylen Dipivalat
20. 4-[1-hydroxy-2-(methylamino)ethyl]-1,2-phenylene Bis(2,2-dimethylpropanoate)
21. (+-)-3,4-dihydroxy-alpha-((methylamino)methyl)benzyl Alcohol 3,4-dipivalate
22. [2-(2,2-dimethylpropanoyloxy)-4-[1-hydroxy-2-(methylamino)ethyl]phenyl] 2,2-dimethylpropanoate
23. Dipivefrin [mi]
24. (rs)-4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylen Dipivalat
25. Prestwick0_000632
26. Prestwick1_000632
27. Prestwick2_000632
28. Prestwick3_000632
29. Dipivefrin [vandf]
30. Dipivefrine [mart.]
31. Propanoic Acid, 2,2-dimethyl-, 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Ester, (+-)-
32. Schembl24713
33. Bspbio_000624
34. Dipivefrine [who-dd]
35. Spbio_002843
36. Bpbio1_000688
37. Gtpl7166
38. Chembl1201262
39. Dtxsid1048544
40. (+-)-4-(1-hydroxy-2-methylaminoethyl)-o-phenylendipivalat
41. (+-)-4-[1-hydroxy-2-(methylamino)ethyl]-o-phenylene Divavalate
42. Db00449
43. (+-)-2,2-dimethylpropansaeure-4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylenester
44. 2,2-dimethylpropanoic Acid 4-[1-hydroxy-2-(methylamino)ethyl]-1,2-phenylene Ester
45. 4-[1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diyl Bis(2,2-dimethylpropanoate)
46. Ncgc00179499-01
47. Ncgc00179499-05
48. Hy-121398
49. Ab00514686
50. Cs-0081941
51. Ft-0745765
52. C06963
53. D02349
54. 365d636
55. L000915
56. Q905952
57. Brd-a47494775-003-03-0
58. (+/-)-4-[1-hydroxy-2-(methylamino)ethyl]-o-phenylene Divavalate
59. Bispivalic Acid 4-[1-hydroxy-2-(methylamino)ethyl]-1,2-phenylene Ester
60. 2-[(2,2-dimethylpropanoyl)oxy]-4-[1-hydroxy-2-(methylamino)ethyl]phenyl 2,2-dimethylpropanoate
61. 2-[(2,2-dimethylpropanoyl)oxy]-5-[1-hydroxy-2-(methylamino)ethyl]phenyl 2,2-dimethylpropanoate
62. Propanoic Acid, 2,2-dimethyl-, 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-phenylene Ester, (+/-)-
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C19H29NO5 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 351.20457303 g/mol |
| Monoisotopic Mass | 351.20457303 g/mol |
| Topological Polar Surface Area | 84.9 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 463 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dipivefrin is a prodrug which is used as initial therapy for the control of intraocular pressure in chronic open-angle glaucoma.
FDA Label
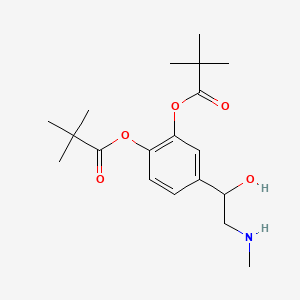
Dipivefrin is a member of a class of drugs known as prodrugs. Prodrugs are usually not active in themselves and require biotransformation to the parent compound before therapeutic activity is seen. These modifications are undertaken to enhance absorption, decrease side effects and enhance stability and comfort, thus making the parent compound a more useful drug. Enhanced absorption makes the prodrug a more efficient delivery system for the parent drug because less drug will be needed to produce the desired therapeutic response. Dipivefrin is a prodrug of epinephrine formed by the diesterification of epinephrine and pivalic acid. The addition of pivaloyl groups to the epinephrine molecule enhances its lipophilic character and, as a consequence, its penetration into the anterior chamber.
Adrenergic Agonists
Drugs that bind to and activate adrenergic receptors. (See all compounds classified as Adrenergic Agonists.)
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EA - Sympathomimetics in glaucoma therapy
S01EA02 - Dipivefrine
Absorption
Well absorbed following occular administration.
Dipivefrin is converted to epinephrine inside the human eye by enzyme hydrolysis.
Dipivefrin is a prodrug with little or no pharmacologically activity until it is hydrolyzed into epinephrine inside the human eye. The liberated epinephrine, an adrenergic agonist, appears to exert its action by stimulating -and/or 2-adrenergic receptors, leading to a decrease in aqueous production and an enhancement of outflow facility. The dipivefrin prodrug delivery system is a more efficient way of delivering the therapeutic effects of epinephrine, with fewer side effects than are associated with conventional epinephrine therapy.